Phase I/II study of docetaxel and S-1 in patients with advanced gastric cancer
- PMID: 16773074
- PMCID: PMC2361339
- DOI: 10.1038/sj.bjc.6603196
Phase I/II study of docetaxel and S-1 in patients with advanced gastric cancer
Abstract
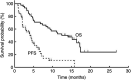
The aims of this phase I/II study of docetaxel and S-1 were to determine the dose-limiting toxicity (DLT), maximum-tolerated dose (MTD), and recommended dose (RD) in the phase I part and to explore the tumour response, survival and safety in the phase II part. Patients with histologically- or cytologically confirmed unresectable or recurrent gastric cancer were eligible. Treatment consisted of intravenous docetaxel on day 1 (starting dose 50 mg m(-2)) and oral S-1 at a fixed dose of 40 mg m(-2) twice daily on days 1-14, every 4 weeks up to six cycles. Nine patients took part in the phase I portion of the study. The MTD of docetaxel was determined to be 50 mg m(-2), with the DLTs of grade 3 infection associated with grade 3 neutropenia and grade 4 neutropenia during S-1 administration. The RD of docetaxel was 40 mg m(-2) in combination with S-1 40 mg m(-2) b.i.d. The efficacy and safety of this regimen was therefore assessed in 46 patients with at least one measurable lesion. The overall response rate and estimated median overall survival were 46% (95% CI, 31-61%) and 14.0 months (8.3-17.3 months), respectively. The most common grade 3/4 toxicity was neutropenia (67% of patients), which was predictable and manageable. This regimen showed promising activity with moderate toxicities in advanced gastric cancer.
Figures
References
-
- Ajani JA, Fodor MB, Tjulandin SA, Moiseyenko VM, Chao Y, Filho SC, Majlis A, Assadourian S, Van Cutsem E (2005) Phase II multi-institutional randomized trial of docetaxel plus cisplatin with or without fluorouracil in patients with untreated, advanced gastric, or gastroesophageal adenocarcinoma. J Clin Oncol 23: 5660–5667 - PubMed
-
- Alberts SR, Cervantes A, van de Velde CJH (2003) Gastric cancer: epidemiology, pathology and treatment. Ann Oncol 14(Suppl 2): ii31–ii36 - PubMed
-
- Bissery MC, Nohynek G, Sanderink GJ, Lavelle F (1995) Docetaxel (Taxotere): a review of preclinical and clinical experience. Part I: preclinical experience. Anticancer Drugs 6: 339–368 - PubMed
-
- Chollet P, Schoffski P, Weigang-Kohler K, Schellens JH, Cure H, Pavlidis N, Grunwald V, De Boer R, Wanders J, Fumoleau P, EORTC Early Clinical Studies Group (2003) Phase II trial with S-1 in chemotherapy-naive patients with gastric cancer. A trial performed by the EORTC Early Clinical Studies Group (ECSG). Eur J Cancer 39: 1264–1270 - PubMed
-
- Constenla M, Garcia-Arroyo R, Lorenzo I, Carrete N, Campos B, Palacios P (2002) Docetaxel, 5-fluorouracil, and leucovorin as treatment for advanced gastric cancer: results of a phase II study. Gastric Cancer 5: 142–147 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous