Pharmacogenetics in inflammatory bowel disease
- PMID: 16773681
- PMCID: PMC4087457
- DOI: 10.3748/wjg.v12.i23.3657
Pharmacogenetics in inflammatory bowel disease
Abstract
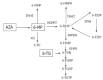
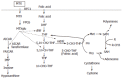
Pharmacogenetics is the study of the association between variability in drug response and (or) drug toxicity and polymorphisms in genes. The goal of this field of science is to adapt drugs to a patient's specific genetic background and therefore make them more efficacious and safe. In this article we describe the variants in genes that influence either the efficacy or toxicity of common drugs used in the treatment of inflammatory bowel diseases (IBD), ulcerative colitis (UC), and Crohn's disease (CD) including sulfasalazine and mesalazine, azathioprine (AZA) and 6-mercaptopurine (6-MP), methotrexate (MTX), glucocorticosteroids (CSs) and infliximab. Furthermore, difficulties with pharmacogenetic studies in general and more specifically in IBD are described. Although pharmacogenetics is a promising field that already contributed to a better understanding of some of the underlying mechanisms of action of drugs used in IBD, the only discovery translated until now into daily practice is the relation between thiopurine S-methyltransferase (TPMT) gene polymorphisms and hematological toxicity of thiopurine treatment. In the future it is necessary to organize studies in well characterized patient cohorts who have been uniformly treated and systematically evaluated in order to quantitate drug response more objectively. An effort should be made to collect genomic DNA from all patients enrolled in clinical drug trials after appropriate informed consent for pharmacogenetic studies.
Figures

References
-
- Travis S, Jewell DP. Ulcerative colitis: clinical presentation and diagnosis. In: Satsangi J and Sutherland LR, eds , editors. Inflammatory Bowel Disease. New York: Churchill Livingstone; 2003. pp. 169–182.
-
- Forbes A. Clinical presentation and diagnosis of Crohn's disease. In: Satsangi J and Sutherland L, eds , editors. Inflammatory Bowel Disease. New York: Churchill Livingstone; 2003. pp. 183–189.
-
- Shibolet O, Regushevskaya E, Brezis M, Soares-Weiser K. Cyclosporine A for induction of remission in severe ulcerative colitis. Cochrane Database Syst Rev. 2005:CD004277. - PubMed
-
- Rutgeerts P, Sandborn WJ, Feagan BG, Reinisch W, Olson A, Johanns J, Travers S, Rachmilewitz D, Hanauer SB, Lichtenstein GR, et al. Infliximab for induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2005;353:2462–2476. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

