Cholangiocyte anion exchange and biliary bicarbonate excretion
- PMID: 16773707
- PMCID: PMC4087566
- DOI: 10.3748/wjg.v12.i22.3496
Cholangiocyte anion exchange and biliary bicarbonate excretion
Abstract
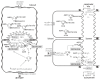
Primary canalicular bile undergoes a process of fluidization and alkalinization along the biliary tract that is influenced by several factors including hormones, innervation/neuropeptides, and biliary constituents. The excretion of bicarbonate at both the canaliculi and the bile ducts is an important contributor to the generation of the so-called bile-salt independent flow. Bicarbonate is secreted from hepatocytes and cholangiocytes through parallel mechanisms which involve chloride efflux through activation of Cl- channels, and further bicarbonate secretion via AE2/SLC4A2-mediated Cl-/HCO3- exchange. Glucagon and secretin are two relevant hormones which seem to act very similarly in their target cells (hepatocytes for the former and cholangiocytes for the latter). These hormones interact with their specific G protein-coupled receptors, causing increases in intracellular levels of cAMP and activation of cAMP-dependent Cl- and HCO3- secretory mechanisms. Both hepatocytes and cholangiocytes appear to have cAMP-responsive intracellular vesicles in which AE2/SLC4A2 colocalizes with cell specific Cl- channels (CFTR in cholangiocytes and not yet determined in hepatocytes) and aquaporins (AQP8 in hepatocytes and AQP1 in cholangiocytes). cAMP-induced coordinated trafficking of these vesicles to either canalicular or cholangiocyte lumenal membranes and further exocytosis results in increased osmotic forces and passive movement of water with net bicarbonate-rich hydrocholeresis.
Figures

References
-
- Boyer JL. Canalicular bile formation in the isolated perfused rat liver. Am J Physiol. 1971;221:1156–1163. - PubMed
-
- Kanno N, LeSage G, Glaser S, Alpini G. Regulation of cholangiocyte bicarbonate secretion. Am J Physiol Gastrointest Liver Physiol. 2001;281:G612–G625. - PubMed
-
- Boyer JL. Bile duct epithelium: frontiers in transport physiology. Am J Physiol. 1996;270:G1–G5. - PubMed
-
- Alpini G, Glaser S, Robertson W, Rodgers RE, Phinizy JL, Lasater J, LeSage GD. Large but not small intrahepatic bile ducts are involved in secretin-regulated ductal bile secretion. Am J Physiol. 1997;272:G1064–G1074. - PubMed
-
- Baiocchi L, LeSage G, Glaser S, Alpini G. Regulation of cholangiocyte bile secretion. J Hepatol. 1999;31:179–191. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

