Inhibition of protein geranylgeranylation induces apoptosis in synovial fibroblasts
- PMID: 16774691
- PMCID: PMC1779395
- DOI: 10.1186/ar1968
Inhibition of protein geranylgeranylation induces apoptosis in synovial fibroblasts
Abstract
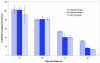
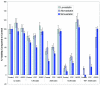
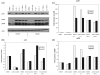
Statins, competitive inhibitors of hydroxymethylglutaryl-CoA reductase, have recently been shown to have a therapeutic effect in rheumatoid arthritis (RA). In RA, synovial fibroblasts in the synovial lining, are believed to be particularly important in the pathogenesis of disease because they recruit leukocytes into the synovium and secrete angiogenesis-promoting molecules and proteases that degrade extracellular matrix. In this study, we show a marked reduction in RA synovial fibroblast survival through the induction of apoptosis when the cells were cultured with statins. Simvastatin was more effective in RA synovial fibroblasts than atorvastatin, and both statins were more potent on tumor necrosis factor-alpha-induced cells. In contrast, in osteoarthritis synovial fibroblasts, neither the statin nor the activation state of the cell contributed to the efficacy of apoptosis induction. Viability of statin-treated cells could be rescued by geranylgeraniol but not by farnesol, suggesting a requirement for a geranylgeranylated protein for synovial fibroblast survival. Phase partitioning experiments confirmed that in the presence of statin, geranylgeranylated proteins are redistributed to the cytoplasm. siRNA experiments demonstrated a role for Rac1 in synovial fibroblast survival. Western blotting showed that the activated phosphorylated form of Akt, a protein previously implicated in RA synovial fibroblast survival, was decreased by about 75%. The results presented in this study lend further support to the importance of elevated pAkt levels to RA synovial fibroblast survival and suggest that statins might have a beneficial role in reducing the aberrant pAkt levels in patients with RA. The results may also partly explain the therapeutic effect of atorvastatin in patients with RA.
Figures



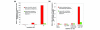
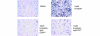
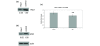
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

