The intraflagellar transport protein IFT20 is associated with the Golgi complex and is required for cilia assembly
- PMID: 16775004
- PMCID: PMC1593158
- DOI: 10.1091/mbc.e06-02-0133
The intraflagellar transport protein IFT20 is associated with the Golgi complex and is required for cilia assembly
Abstract
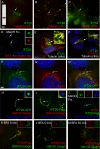
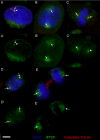
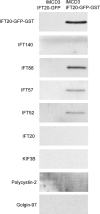
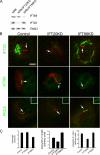
Eukaryotic cilia are assembled via intraflagellar transport (IFT) in which large protein particles are motored along ciliary microtubules. The IFT particles are composed of at least 17 polypeptides that are thought to contain binding sites for various cargos that need to be transported from their site of synthesis in the cell body to the site of assembly in the cilium. We show here that the IFT20 subunit of the particle is localized to the Golgi complex in addition to the basal body and cilia where all previous IFT particle proteins had been found. In living cells, fluorescently tagged IFT20 is highly dynamic and moves between the Golgi complex and the cilium as well as along ciliary microtubules. Strong knock down of IFT20 in mammalian cells blocks ciliary assembly but does not affect Golgi structure. Moderate knockdown does not block cilia assembly but reduces the amount of polycystin-2 that is localized to the cilia. This work suggests that IFT20 functions in the delivery of ciliary membrane proteins from the Golgi complex to the cilium.
Figures


References
-
- Allan V. J., Thompson H. M., McNiven M. A. Motoring around the Golgi. Nat. Cell Biol. 2002;4:E236–E242. - PubMed
-
- Baker S. A., Freeman K., Luby-Phelps K., Pazour G. J., Besharse J. C. IFT20 links kinesin II with a mammalian intraflagellar transport complex that is conserved in motile flagella and sensory cilia. J. Biol. Chem. 2003;278:34211–34218. - PubMed
-
- Bloodgood R. A. Ciliary and Flagellar Membranes. New York: Plenum Press; 1990.
-
- Brailov I., Bancila M., Brisorgueil M., Miquel M., Hamon M., Verge D. Localization of 5-HT(6) receptors at the plasma membrane of neuronal cilia in the rat brain. Brain Res. 2000;872:271–275. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

