Phosphoinositide 3-Kinase C2beta regulates cytoskeletal organization and cell migration via Rac-dependent mechanisms
- PMID: 16775008
- PMCID: PMC1593155
- DOI: 10.1091/mbc.e05-11-1083
Phosphoinositide 3-Kinase C2beta regulates cytoskeletal organization and cell migration via Rac-dependent mechanisms
Abstract
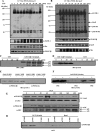
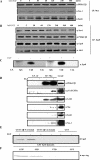
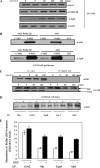
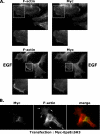
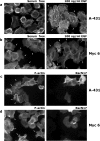
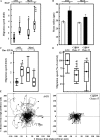
Receptor-linked class I phosphoinositide 3-kinases (PI3Ks) induce assembly of signal transduction complexes through protein-protein and protein-lipid interactions that mediate cell proliferation, survival, and migration. Although class II PI3Ks have the potential to make the same phosphoinositides as class I PI3Ks, their precise cellular role is currently unclear. In this report, we demonstrate that class II phosphoinositide 3-kinase C2beta (PI3KC2beta) associates with the Eps8/Abi1/Sos1 complex and is recruited to the EGF receptor as part of a multiprotein signaling complex also involving Shc and Grb2. Increased expression of PI3KC2beta stimulated Rac activity in A-431 epidermoid carcinoma cells, resulting in enhanced membrane ruffling and migration speed of the cells. Conversely, expression of dominant negative PI3KC2beta reduced Rac activity, membrane ruffling, and cell migration. Moreover, PI3KC2beta-overexpressing cells were protected from anoikis and displayed enhanced proliferation, independently of Rac function. Taken together, these findings suggest that PI3KC2beta regulates the migration and survival of human tumor cells by distinct molecular mechanisms.
Figures



Similar articles
-
Phosphoinositide 3-kinase activates Rac by entering in a complex with Eps8, Abi1, and Sos-1.J Cell Biol. 2003 Jan 6;160(1):17-23. doi: 10.1083/jcb.200206079. Epub 2003 Jan 6. J Cell Biol. 2003. PMID: 12515821 Free PMC article.
-
Phosphoinositide 3-kinase C2β regulates RhoA and the actin cytoskeleton through an interaction with Dbl.PLoS One. 2012;7(9):e44945. doi: 10.1371/journal.pone.0044945. Epub 2012 Sep 12. PLoS One. 2012. PMID: 22984590 Free PMC article.
-
Integrity of SOS1/EPS8/ABI1 tri-complex determines ovarian cancer metastasis.Cancer Res. 2010 Dec 1;70(23):9979-90. doi: 10.1158/0008-5472.CAN-10-2394. Epub 2010 Nov 30. Cancer Res. 2010. PMID: 21118970 Free PMC article.
-
Phosphoinositide 3-kinase-regulated adapters in lymphocyte activation.Immunol Rev. 2009 Nov;232(1):255-72. doi: 10.1111/j.1600-065X.2009.00838.x. Immunol Rev. 2009. PMID: 19909369 Review.
-
Eps8 in the midst of GTPases.Int J Biochem Cell Biol. 2002 Oct;34(10):1178-83. doi: 10.1016/s1357-2725(02)00064-x. Int J Biochem Cell Biol. 2002. PMID: 12127568 Review.
Cited by
-
Crossroads of PI3K and Rac pathways.Small GTPases. 2015;6(2):71-80. doi: 10.4161/21541248.2014.989789. Epub 2015 May 5. Small GTPases. 2015. PMID: 25942647 Free PMC article. Review.
-
CIIA functions as a molecular switch for the Rac1-specific GEF activity of SOS1.J Cell Biol. 2011 Oct 31;195(3):377-86. doi: 10.1083/jcb.201106138. J Cell Biol. 2011. PMID: 22042618 Free PMC article.
-
Amplification of Chromosome 1q Genes Encoding the Phosphoinositide Signalling Enzymes PI4KB, AKT3, PIP5K1A and PI3KC2B in Breast Cancer.J Cancer. 2014 Oct 28;5(9):790-6. doi: 10.7150/jca.9794. eCollection 2014. J Cancer. 2014. PMID: 25368680 Free PMC article.
-
Class II phosphoinositide 3-kinases contribute to endothelial cells morphogenesis.PLoS One. 2013;8(1):e53808. doi: 10.1371/journal.pone.0053808. Epub 2013 Jan 8. PLoS One. 2013. PMID: 23320105 Free PMC article.
-
Phosphoinositide 3-kinase signalling in lung disease: leucocytes and beyond.Immunology. 2007 Aug;121(4):448-61. doi: 10.1111/j.1365-2567.2007.02663.x. Immunology. 2007. PMID: 17614878 Free PMC article. Review.
References
-
- Arcaro A., Volinia S., Zvelebil M. J., Stein R., Watton S. J., Layton M. J., Gout I., Ahmadi K., Downward J., Waterfield M. D. Human phosphoinositide 3-kinase C2beta, the role of calcium and the C2 domain in enzyme activity. J. Biol. Chem. 1998;273:33082–33090. - PubMed
-
- Bapat S., Verkleij A., Post J. A. Peroxynitrite activates mitogen-activated protein kinase (MAPK) via a MEK-independent pathway: a role for PKC. FEBS Lett. 2001;499:21–26. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

