Subventricular zone-derived neuroblasts migrate and differentiate into mature neurons in the post-stroke adult striatum
- PMID: 16775151
- PMCID: PMC6674034
- DOI: 10.1523/JNEUROSCI.0149-06.2006
Subventricular zone-derived neuroblasts migrate and differentiate into mature neurons in the post-stroke adult striatum
Abstract
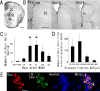
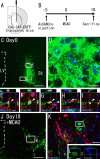
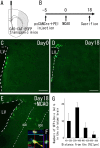
Recent studies have revealed that the adult mammalian brain has the capacity to regenerate some neurons after various insults. However, the precise mechanism of insult-induced neurogenesis has not been demonstrated. In the normal brain, GFAP-expressing cells in the subventricular zone (SVZ) of the lateral ventricles include a neurogenic cell population that gives rise to olfactory bulb neurons only. Herein, we report evidence that, after a stroke, these cells are capable of producing new neurons outside the olfactory bulbs. SVZ GFAP-expressing cells labeled by a cell-type-specific viral infection method were found to generate neuroblasts that migrated toward the injured striatum after middle cerebral artery occlusion. These neuroblasts in the striatum formed elongated chain-like cell aggregates similar to those in the normal SVZ, and these chains were observed to be closely associated with thin astrocytic processes and blood vessels. Finally, long-term tracing of the green fluorescent-labeled cells with a Cre-loxP system revealed that the SVZ-derived neuroblasts differentiated into mature neurons in the striatum, in which they expressed neuronal-specific nuclear protein and formed synapses with neighboring striatal cells. These results highlight the role of the SVZ in neuronal regeneration after a stroke and its potential as an important therapeutic target for various neurological disorders.
Figures



References
-
- Altman J (1969). Autoradiographic and histological studies of postnatal neurogenesis. IV. Cell proliferation and migration in the anterior forebrain, with special reference to persisting neurogenesis in the olfactory bulb. J Comp Neurol 137:433–457. - PubMed
-
- Araki T, Shibata M, Takano R, Hisahara S, Imamura S, Fukuuchi Y, Saruta T, Okano H, Miura M (2000). Conditional expression of anti-apoptotic protein p35 by Cre-mediated DNA recombination in cardiomyocytes from loxP-p35-transgenic mice. Cell Death Differ 7:485–492. - PubMed
-
- Arvidsson A, Kokaia Z, Lindvall O (2001). N-methyl-d-aspartate receptor-mediated increase of neurogenesis in adult rat dentate gyrus following stroke. Eur J Neurosci 14:10–18. - PubMed
-
- Arvidsson A, Collin T, Kirik D, Kokaia Z, Lindvall O (2002). Neuronal replacement from endogenous precursors in the adult brain after stroke. Nat Med 8:963–970. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
