Herpes simplex virus capsid structure: DNA packaging protein UL25 is located on the external surface of the capsid near the vertices
- PMID: 16775316
- PMCID: PMC1488932
- DOI: 10.1128/JVI.02648-05
Herpes simplex virus capsid structure: DNA packaging protein UL25 is located on the external surface of the capsid near the vertices
Abstract
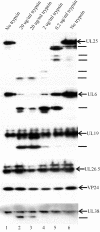
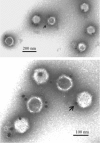
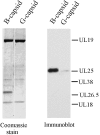
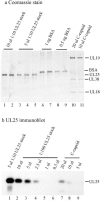
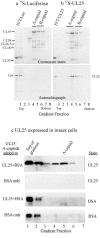
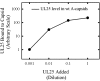
UL25 is one of seven herpes simplex virus-encoded proteins involved specifically in DNA encapsidation. Its role appears to be to stabilize the capsid so that DNA is prevented from escaping once it has entered. To clarify the function of UL25, we have examined capsids with the goal of defining where it is located. Analysis of trypsin-treated capsids showed that UL25 is sensitive to cleavage like other proteins such as the major capsid and portal proteins that are exposed on the capsid surface. Internal proteins such as the scaffolding protein and protease were not affected under the same experimental conditions. Capsids were also examined by electron microscopy after staining with gold-labeled antibody specific for UL25. Images of stained capsids demonstrated that most labeled sites (71% in C capsids) were at capsid vertices, and most stained C capsids had label at more than one vertex. A quantitative immunoblotting method showed that the capsid contents of UL25 were 56, 20, and 75 copies per capsid in A, B, and C capsids, respectively. Finally, soluble UL25 protein was found to bind in vitro to purified capsids lacking it. The amount of bound UL25 corresponded to the amount present in B capsids, and bound UL25 was found by immunoelectron microscopy to be located predominantly at the capsid vertices. The results are interpreted to suggest that five UL25 molecules are found at or near each of the capsid vertices, where they are exposed on the capsid surface. Exposure on the surface is consistent with the view that UL25 is added to the capsid as DNA is packaged or during late stages of the packaging process.
Figures
References
-
- Aebi, A., R. van Driel, R. K. L. Bijlenga, B. ten Heggeler, R. van der Broek, A. C. Steven, and P. R. Smith. 1977. Capsid fine structure of T-even bacteriophages. Binding and localization of two dispensable capsid proteins into the P23 surface lattice. J. Mol. Biol. 110:687-698. - PubMed
-
- Al-Kobaisi, M. F., F. J. Rixon, I. McDougall, and V. G. Preston. 1991. The herpes simplex virus UL33 gene product is required for the assembly of full capsids. Virology 180:380-388. - PubMed
-
- Brown, J. C., M. A. McVoy, and F. L. Homa. 2002. Packaging DNA into herpesvirus capsids, p. 111-153. In A. Holzenburg and E. Bogner (ed.), Structure-function relationships of human pathogenic viruses. Kluwer Academic/Plenum Publishers, London, United Kingdom.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

