Influenza virus evades innate and adaptive immunity via the NS1 protein
- PMID: 16775317
- PMCID: PMC1488970
- DOI: 10.1128/JVI.02381-05
Influenza virus evades innate and adaptive immunity via the NS1 protein
Abstract
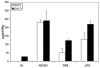
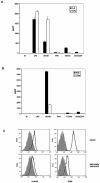
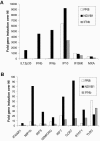
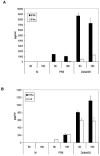
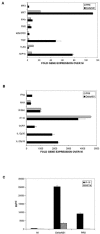
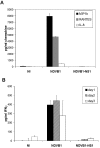
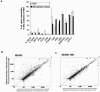
Both antibodies and T cells contribute to immunity against influenza virus infection. However, the generation of strong Th1 immunity is crucial for viral clearance. Interestingly, we found that human dendritic cells (DCs) infected with influenza A virus have lower allospecific Th1-cell stimulatory abilities than DCs activated by other stimuli, such as lipopolysaccharide and Newcastle disease virus infection. This weak stimulatory activity correlates with a suboptimal maturation of the DCs following infection with influenza A virus. We next investigated whether the influenza A virus NS1 protein could be responsible for the low levels of DC maturation after influenza virus infection. The NS1 protein is an important virulence factor associated with the suppression of innate immunity via the inhibition of type I interferon (IFN) production in infected cells. Using recombinant influenza and Newcastle disease viruses, with or without the NS1 gene from influenza virus, we found that the induction of a genetic program underlying DC maturation, migration, and T-cell stimulatory activity is specifically suppressed by the expression of the NS1 protein. Among the genes affected by NS1 are those coding for macrophage inflammatory protein 1beta, interleukin-12 p35 (IL-12 p35), IL-23 p19, RANTES, IL-8, IFN-alpha/beta, and CCR7. These results indicate that the influenza A virus NS1 protein is a bifunctional viral immunosuppressor which inhibits innate immunity by preventing type I IFN release and inhibits adaptive immunity by attenuating human DC maturation and the capacity of DCs to induce T-cell responses. Our observations also support the potential use of NS1 mutant influenza viruses as live attenuated influenza virus vaccines.
Figures

References
-
- Banchereau, J., F. Briere, C. Caux, J. Davoust, S. Lebecque, Y. J. Liu, B. Pulendran, and K. Palucka. 2000. Immunobiology of dendritic cells. Annu. Rev. Immunol. 18:767-811. - PubMed
-
- Banchereau, J., and R. M. Steinman. 1998. Dendritic cells and the control of immunity. Nature 392:245-252. - PubMed
-
- Barchet, W., A. Krug, M. Cella, C. Newby, J. A. Fischer, A. Dzionek, A. Pekosz, and M. Colonna. 2005. Dendritic cells respond to influenza virus through TLR7- and PKR-independent pathways. Eur. J. Immunol. 35:236-242. - PubMed
-
- Belladonna, M. L., J. C. Renauld, R. Bianchi, C. Vacca, F. Fallarino, C. Orabona, M. C. Fioretti, U. Grohmann, and P. Puccetti. 2002. IL-23 and IL-12 have overlapping, but distinct, effects on murine dendritic cells. J. Immunol. 168:5448-5454. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

