Non-macrophage-tropic human immunodeficiency virus type 1 R5 envelopes predominate in blood, lymph nodes, and semen: implications for transmission and pathogenesis
- PMID: 16775320
- PMCID: PMC1488974
- DOI: 10.1128/JVI.02328-05
Non-macrophage-tropic human immunodeficiency virus type 1 R5 envelopes predominate in blood, lymph nodes, and semen: implications for transmission and pathogenesis
Abstract
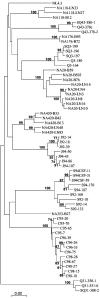
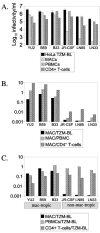
Human immunodeficiency virus type 1 (HIV-1) R5 isolates that predominantly use CCR5 as a coreceptor are frequently described as macrophage tropic. Here, we compare macrophage tropism conferred by HIV-1 R5 envelopes that were derived directly by PCR from patient tissue. This approach avoids potentially selective culture protocols used in virus isolation. Envelopes were amplified (i) from blood and semen of adult patients and (ii) from plasma of pediatric patients. The phenotypes of these envelopes were compared to those conferred by an extended panel of envelopes derived from brain and lymph node that we reported previously. Our results show that R5 envelopes vary by up to 1,000-fold in their capacity to confer infection of primary macrophages. Highly macrophage-tropic envelopes were predominate in brain but were infrequent in semen, blood, and lymph node samples. We also confirmed that the presence of N283 in the C2 CD4 binding site of gp120 is associated with HIV-1 envelopes from the brain but absent from macrophage-tropic envelopes amplified from blood and semen. Finally, we compared infection of macrophages, CD4(+) T cells, and peripheral blood mononuclear cells (PBMCs) conferred by macrophage-tropic and non-macrophage-tropic envelopes in the context of full-length replication competent viral clones. Non-macrophage-tropic envelopes conferred low-level infection of macrophages yet infected CD4(+) T cells and PBMCs as efficiently as highly macrophage-tropic brain envelopes. The lack of macrophage tropism for the majority of the envelopes amplified from lymph node, blood, and semen is striking and contrasts with the current consensus that R5 primary isolates are generally macrophage tropic. The extensive variation in R5 tropism reported here is likely to have an important impact on pathogenesis and on the capacity of HIV-1 to transmit.
Figures


References
-
- Asjo, B., L. Morfeldt Manson, J. Albert, G. Biberfeld, A. Karlsson, K. Lidman, and E. M. Fenyo. 1986. Replicative capacity of human immunodeficiency virus from patients with varying severity of HIV infection. Lancet ii:660-662. - PubMed
-
- Berger, E. A., R. W. Doms, E.-M. Fenyo, B. T. M. Korber, D. R. Littman, J. P. Moore, Q. J. Sattentau, H. Schuitemaker, J. Sodroski, and R. A. Weiss. 1998. A new classification for HIV-1. Nature 391:240. - PubMed
-
- Blaak, H., A. B. van't Wout, M. Brouwer, B. Hooibrink, E. Hovenkamp, and H. Schuitemaker. 2000. In vivo HIV-1 infection of CD45RA+CD4+ T cells is established primarily by syncytium-inducing variants and correlates with the rate of CD4+ T cell decline. Proc. Natl. Acad. Sci. USA 97:1269-1274. - PMC - PubMed
-
- Bounou, S., J. F. Giguere, R. Cantin, C. Gilbert, M. Imbeault, G. Martin, and M. J. Tremblay. 2004. The importance of virus-associated host ICAM-1 in human immunodeficiency virus type 1 dissemination depends on the cellular context. FASEB J. 18:1294-1296. - PubMed
-
- Carrington, M., M. Dean, M. P. Martin, and S. J. O'Brien. 1999. Genetics of HIV-1 infection: chemokine receptor CCR5 polymorphism and its consequences. Hum. Mol. Genet. 8:1939-1945. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

