Simian virus 40 late proteins possess lytic properties that render them capable of permeabilizing cellular membranes
- PMID: 16775344
- PMCID: PMC1488938
- DOI: 10.1128/JVI.00347-06
Simian virus 40 late proteins possess lytic properties that render them capable of permeabilizing cellular membranes
Abstract
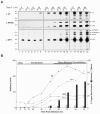
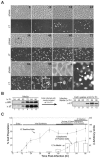
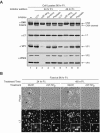
Many nonenveloped viruses have evolved an infectious cycle that culminates in the lysis or permeabilization of the host to enable viral release. How these viruses initiate the lytic event is largely unknown. Here, we demonstrated that the simian virus 40 progeny accumulated at the nuclear envelope prior to the permeabilization of the nuclear, endoplasmic reticulum, and plasma membranes at a time which corresponded with the release of the progeny. The permeabilization of these cellular membranes temporally correlated with late protein expression and was not observed upon the inhibition of their synthesis. To address whether one or more of the late proteins possessed an inherent capacity to induce membrane permeabilization, we examined the permeability of Escherichia coli that separately expressed the late proteins. VP2 and VP3, but not VP1, caused the permeabilization of bacterial membranes. Additionally, VP3 expression resulted in bacterial cell lysis. These findings demonstrate that VP3 possesses an inherent lytic property that is independent of eukaryotic signaling or cell death pathways.
Figures




References
-
- Berger, N. A. 1985. Poly(ADP-ribose) in the cellular response to DNA damage. Radiat. Res. 101:4-15. - PubMed
-
- Brostrom, M. A., C. R. Prostko, D. Gmitter-Yellen, L. J. Grandison, G. Kuznetsov, W. L. Wong, and C. O. Brostrom. 1991. Inhibition of translational initiation by metalloendoprotease antagonists. Evidence for involvement of sequestered Ca2+ stores. J. Biol. Chem. 266:7037-7043. - PubMed
-
- Burkle, A. 2005. Poly(ADP-ribose). The most elaborate metabolite of NAD+. FEBS J. 272:4576-4589. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

