Identification of a dynein interacting domain in the papillomavirus minor capsid protein l2
- PMID: 16775357
- PMCID: PMC1488977
- DOI: 10.1128/JVI.00057-06
Identification of a dynein interacting domain in the papillomavirus minor capsid protein l2
Abstract
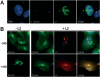
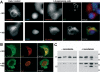
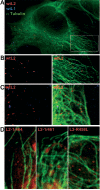
Papillomaviruses enter cells via endocytosis (H. C. Selinka et al., Virology 299:279-287, 2002). After egress from endosomes, the minor capsid protein L2 accompanies the viral DNA to the nucleus and subsequently to the subnuclear promyelocytic leukemia protein bodies (P. M. Day et al., Proc. Natl. Acad. Sci. USA 101:14252-14257, 2004), suggesting that this protein may be involved in the intracytoplasmic transport of the viral genome. We now demonstrate that the L2 protein is able to interact with the microtubule network via the motor protein dynein. L2 protein was found attached to microtubules after uncoating of incoming human papillomavirus pseudovirions. Based on immunofluorescence and coimmunoprecipitation analyses, the L2 region interacting with dynein is mapped to the C-terminal 40 amino acids. Mutations within this region abrogating the L2/dynein interaction strongly reduce the infectivity of pseudoviruses, indicating that this interaction mediates the minus-end-directed transport of the viral genome along microtubules towards the nucleus.
Figures


References
-
- Becker, K. A., L. Florin, C. Sapp, and M. Sapp. 2003. Dissection of human papillomavirus type 33 L2 domains involved in nuclear domain (ND) 10 homing and reorganization. Virology 314:161-167. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

