The tripeptide feG regulates the production of intracellular reactive oxygen species by neutrophils
- PMID: 16776845
- PMCID: PMC1534017
- DOI: 10.1186/1476-9255-3-9
The tripeptide feG regulates the production of intracellular reactive oxygen species by neutrophils
Abstract
Background: The D-isomeric form of the tripeptide FEG (feG) is a potent anti-inflammatory agent that suppresses type I hypersensitivity (IgE-mediated allergic) reactions in several animal species. One of feG's primary actions is to inhibit leukocyte activation resulting in loss of their adhesive and migratory properties. Since activation of neutrophils is often associated with an increase in respiratory burst with the generation of reactive oxygen species (ROS), we examined the effect of feG on the respiratory burst in neutrophils of antigen-sensitized rats. A role for protein kinase C (PKC) in the actions of feG was evaluated by using selective isoform inhibitors for PKC.
Results: At 18 h after antigen (ovalbumin) challenge of sensitized Sprague-Dawley rats a pronounced neutrophilia occurred; a response that was reduced in animals treated with feG (100 microg/kg). With antigen-challenged animals the protein kinase C (PKC) activator, PMA, significantly increased intracellular ROS of circulating neutrophils, as determined by flow cytometry using the fluorescent probe dihydrorhodamine-123. This increase was prevented by treatment with feG at the time of antigen challenge. The inhibitor of PKCdelta, rottlerin, which effectively prevented intracellular ROS production by circulating neutrophils of animals receiving a naïve antigen, failed to inhibit PMA-stimulated ROS production if the animals were challenged with antigen. feG treatment, however, re-established the inhibitory effects of the PKCdelta inhibitor on intracellular ROS production. The extracellular release of superoxide anion, evaluated by measuring the oxidative reduction of cytochrome C, was neither modified by antigen challenge nor feG treatment. However, hispidin, an inhibitor of PKCbeta, inhibited the release of superoxide anion from circulating leukocytes in all groups of animals. feG prevented the increased expression of the beta1-integrin CD49d on the circulating neutrophils elicited by antigen challenge.
Conclusion: feG reduces the capacity of circulating neutrophils to generate intracellular ROS consequent to an allergic reaction by preventing the deregulation of PKCdelta. This action of feG may be related to the reduction in antigen-induced up-regulation of CD49d expression on circulating neutrophils.
Figures

 ; n = 11), or OA + feG (
; n = 11), or OA + feG ( ; n = 13). Challenge with sensitizing antigen (OA) increased the total number of circulating leukocytes, and this increase was prevented by feG (a). Antigen challenge increased significantly the percentage of circulating neutrophils (b), which is reflected in a dramatic increase in the total number of circulating neutrophils (c). These changes elicited by antigen challenge were inhibited significantly by feG. Significance: # > BSA; ## > feG;* < OA
; n = 13). Challenge with sensitizing antigen (OA) increased the total number of circulating leukocytes, and this increase was prevented by feG (a). Antigen challenge increased significantly the percentage of circulating neutrophils (b), which is reflected in a dramatic increase in the total number of circulating neutrophils (c). These changes elicited by antigen challenge were inhibited significantly by feG. Significance: # > BSA; ## > feG;* < OA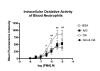
 ; and rottlerin/PKCδ
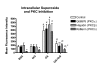
; and rottlerin/PKCδ ) on PMA-stimulated (3.5 × 10-6M) ROS production by circulating neutrophils. Oxidative activity of circulating neutrophils 18 hours after administering to sensitized rats either BSA (n = 5); feG (n = 6); OA antigen (n = 6), or OA + feG (n = 6). Oxidative activity was measured by determining mean fluorescence intensity (MFI) using flow cytometry for a marker of oxygen free radicals (123-dihydrorhodamine; DHR). Significance: * < Control; # > BSA; σ < OA
) on PMA-stimulated (3.5 × 10-6M) ROS production by circulating neutrophils. Oxidative activity of circulating neutrophils 18 hours after administering to sensitized rats either BSA (n = 5); feG (n = 6); OA antigen (n = 6), or OA + feG (n = 6). Oxidative activity was measured by determining mean fluorescence intensity (MFI) using flow cytometry for a marker of oxygen free radicals (123-dihydrorhodamine; DHR). Significance: * < Control; # > BSA; σ < OA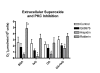
 ; and rottlerin/PKCδ
; and rottlerin/PKCδ ) on PMA (3.5 × 10-6M)-stimulated superoxide release from circulating neutrophils. Oxidative activity was measured 18 hours after administering to ovalbumin (OA)-sensitized rats naïve bovine serum albumin (BSA) (n = 5), sensitizing OA antigen (n = 6), feG (n = 4), or OA + feG (n = 6). Oxidative activity was measured by determined by reduction of cytochrome C. The results are expressed as μmoles/min/106 neutrophils. Significance: * < Control; # > Control.
) on PMA (3.5 × 10-6M)-stimulated superoxide release from circulating neutrophils. Oxidative activity was measured 18 hours after administering to ovalbumin (OA)-sensitized rats naïve bovine serum albumin (BSA) (n = 5), sensitizing OA antigen (n = 6), feG (n = 4), or OA + feG (n = 6). Oxidative activity was measured by determined by reduction of cytochrome C. The results are expressed as μmoles/min/106 neutrophils. Significance: * < Control; # > Control.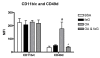
 ; n = 6), or OA + feG (
; n = 6), or OA + feG ( ; n = 6) 18 h before harvesting the cells. Significance: # > BSA; * < OA.
; n = 6) 18 h before harvesting the cells. Significance: # > BSA; * < OA.References
-
- Mathison RD, Befus AD, Davison JS. A novel submandibular gland peptide protects against endotoxic and anaphylactic shock. Am J Physiol. 1997;273:R1017–23. - PubMed
LinkOut - more resources
Full Text Sources

