Thalamic T-type Ca2+ channels and NREM sleep
- PMID: 16777223
- PMCID: PMC3018590
- DOI: 10.1016/j.ceca.2006.04.022
Thalamic T-type Ca2+ channels and NREM sleep
Abstract
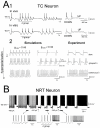
T-type Ca2+ channels play a number of different and pivotal roles in almost every type of neuronal oscillation expressed by thalamic neurones during non-rapid eye movement (NREM) sleep, including those underlying sleep theta waves, the K-complex and the slow (<1 Hz) sleep rhythm, sleep spindles and delta waves. In particular, the transient opening of T channels not only gives rise to the 'classical' low threshold Ca2+ potentials, and associated high frequency burst of action potentials, that are characteristically present during sleep spindles and delta waves, but also contributes to the high threshold bursts that underlie the thalamic generation of sleep theta rhythms. The persistent opening of a small fraction of T channels, i.e. I(Twindow), is responsible for the large amplitude and long lasting depolarization, or UP state, of the slow (<1 Hz) sleep oscillation in thalamic neurones. These cellular findings are in part matched by the wake-sleep phenotype of global and thalamic-selective CaV3.1 knockout mice that show a decreased amount of total NREM sleep time. T-type Ca2+ channels, therefore, constitute the single most crucial voltage-dependent conductance that permeates all activities of thalamic neurones during NREM sleep. Since I(Twindow) and high threshold bursts are not restricted to thalamic neurones, the cellular neurophysiology of T channels should now move away from the simplistic, though historically significant, view of these channels as being responsible only for low threshold Ca2+ potentials.
Figures






References
-
- Llinas R, Jahnsen H. Electrophysiology of mammalian thalamic neurones in vitro. Nature. 1982;297:406–408. - PubMed
-
- Andersen P, Andersson SA. Physiological basis of the alpha rhythm. Century Crofts; New York: 1968.
-
- Deschenes M, Paradis M, Roy JP, Steriade M. Electrophysiology of neurons of lateral thalamic nuclei in cat: resting properties and burst discharges. J. Neurophysiol. 1984;51:1196–1219. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

