Angiotensin II regulates neuronal excitability via phosphatidylinositol 4,5-bisphosphate-dependent modulation of Kv7 (M-type) K+ channels
- PMID: 16777936
- PMCID: PMC1819424
- DOI: 10.1113/jphysiol.2006.114074
Angiotensin II regulates neuronal excitability via phosphatidylinositol 4,5-bisphosphate-dependent modulation of Kv7 (M-type) K+ channels
Abstract
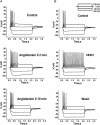
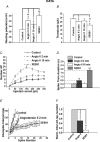
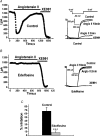
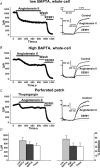
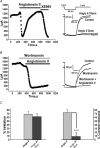
Voltage-gated Kv7 (KCNQ) channels underlie important K+ currents in many different types of cells, including the neuronal M current, which is thought to be modulated by muscarinic stimulation via depletion of membrane phosphatidylinositol 4,5-bisphosphate (PIP2). We studied the role of modulation by angiotensin II (angioII) of M current in controlling discharge properties of superior cervical ganglion (SCG) sympathetic neurons and the mechanism of action of angioII on cloned Kv7 channels in a heterologous expression system. In SCG neurons, which endogenously express angioII AT1 receptors, application of angioII for 2 min produced an increase in neuronal excitability and a decrease in spike-frequency adaptation that partially returned to control values after 10 min of angioII exposure. The increase in excitability could be simulated in a computational model by varying only the amount of M current. Using Chinese hamster ovary (CHO) cells expressing cloned Kv7.2 + 7.3 heteromultimers and AT1 receptors studied under perforated patch clamp, angioII induced a strong suppression of the Kv7.2/7.3 current that returned to near baseline within 10 min of stimulation. The suppression was blocked by the phospholipase C inhibitor edelfosine. Under whole-cell clamp, angioII moderately suppressed the Kv7.2/7.3 current whether or not intracellular Ca2+ was clamped or Ca2+ stores depleted. Co-expression of PI(4)5-kinase in these cells sharply reduced angioII inhibition, but did not augment current amplitudes, whereas co-expression of a PIP2 5'-phosphatase sharply reduced current amplitudes, and also blunted the inhibition. The rebound of the current seen in perforated-patch recordings was blocked by the PI4-kinase inhibitor, wortmannin (50 microM), suggesting that PIP2 re-synthesis is required for current recovery. High-performance liquid chromatographic analysis of anionic phospholipids in CHO cells stably expressing AT1 receptors revealed that PIP2 and phosphatidylinositol 4-phosphate levels are to be strongly depleted after 2 min of stimulation with angioII, with a partial rebound after 10 min. The results of this study establish how angioII modulates M channels, which in turn affects the integrative properties of SCG neurons.
Figures


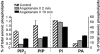
References
-
- Aileru AA, Logan E, Callahan M, Ferrario CM, Ganten D, Diz DI. Alterations in sympathetic ganglionic transmission in response to angiotensin II in (mRen2)27 transgenic rats. Hypertension. 2004;43:270–275. - PubMed
-
- Akasu T, Hirai K, Koketsu K. Modulatory actions of ATP on membrane potentials of bullfrog sympathetic ganglion cells. Brain Res. 1983;258:313–317. - PubMed
-
- Alkadhi KA, Alzoubi KH, Aleisa AM. Plasticity of synaptic transmission in autonomic ganglia. Prog Neurobiol. 2005;75:83–108. - PubMed
-
- Bender K, Wellner-Kienitz MC, Pott L. Transfection of a phosphatidyl-4-phosphate 5-kinase gene into rat atrial myocytes removes inhibition of GIRK current by endothelin and alpha-adrenergic agonists. FEBS Lett. 2002;529:356–360. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

