Characterization of the class IV homeodomain-Leucine Zipper gene family in Arabidopsis
- PMID: 16778018
- PMCID: PMC1533922
- DOI: 10.1104/pp.106.077388
Characterization of the class IV homeodomain-Leucine Zipper gene family in Arabidopsis
Abstract
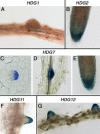
The Arabidopsis (Arabidopsis thaliana) genome contains 16 genes belonging to the class IV homeodomain-Leucine zipper gene family. These include GLABRA2, ANTHOCYANINLESS2, FWA, ARABIDOPSIS THALIANA MERISTEM LAYER1 (ATML1), and PROTODERMAL FACTOR2 (PDF2). Our previous study revealed that atml1 pdf2 double mutants have severe defects in the shoot epidermal cell differentiation. Here, we have characterized additional members of this gene family, which we designated HOMEODOMAIN GLABROUS1 (HDG1) through HDG12. Analyses of transgenic Arabidopsis plants carrying the gene-specific promoter fused to the bacterial beta-glucuronidase reporter gene revealed that some of the promoters have high activities in the epidermal layer of the shoot apical meristem and developing shoot organs, while others are temporarily active during reproductive organ development. Expression profiles of highly conserved paralogous gene pairs within the family were found to be not necessarily overlapping. Analyses of T-DNA insertion mutants of these HDG genes revealed that all mutants except hdg11 alleles exhibit no abnormal phenotypes. hdg11 mutants show excess branching of the trichome. This phenotype is enhanced in hdg11 hdg12 double mutants. Double mutants were constructed for other paralogous gene pairs and genes within the same subfamily. However, novel phenotypes were observed only for hdg3 atml1 and hdg3 pdf2 mutants that both exhibited defects in cotyledon development. These observations suggest that some of the class IV homeodomain-Leucine zipper members act redundantly with other members of the family during various aspects of cell differentiation. DNA-binding sites were determined for two of the family members using polymerase chain reaction-assisted DNA selection from random oligonucleotides with their recombinant proteins. The binding sites were found to be similar to those previously identified for ATML1 and PDF2, which correspond to the pseudopalindromic sequence 5'-GCATTAAATGC-3' as the preferential binding site.
Figures









References
-
- Abe M, Katsumata H, Komeda Y, Takahashi T (2003) Regulation of shoot epidermal cell differentiation by a pair of homeodomain proteins in Arabidopsis. Development 130: 635–643 - PubMed
-
- Abe M, Takahashi T, Komeda Y (2001) Identification of a cis-regulatory element for L1 layer-specific gene expression, which is targeted by an L1-specific homeodomain protein. Plant J 26: 487–494 - PubMed
-
- Becraft PW, Stinard PS, McCarty DR (1996) CRINKLY4: a TNFR-like receptor kinase involved in maize epidermal differentiation. Science 273: 1406–1409 - PubMed
-
- Bowman JL (2004) Class III HD-Zip gene regulation, the golden fleece of ARGONAUTE activity? Bioessays 26: 938–942 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

