Adaptor functions of Cdc42, Ste50, and Sho1 in the yeast osmoregulatory HOG MAPK pathway
- PMID: 16778768
- PMCID: PMC1500976
- DOI: 10.1038/sj.emboj.7601192
Adaptor functions of Cdc42, Ste50, and Sho1 in the yeast osmoregulatory HOG MAPK pathway
Abstract
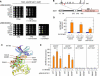
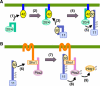
The yeast high osmolarity glycerol (HOG) signaling pathway can be activated by either of the two upstream pathways, termed the SHO1 and SLN1 branches. When stimulated by high osmolarity, the SHO1 branch activates an MAP kinase module composed of the Ste11 MAPKKK, the Pbs2 MAPKK, and the Hog1 MAPK. To investigate how osmostress activates this MAPK module, we isolated both gain-of-function and loss-of-function alleles in four key genes involved in the SHO1 branch, namely SHO1, CDC42, STE50, and STE11. These mutants were characterized using an HOG-dependent reporter gene, 8xCRE-lacZ. We found that Cdc42, in addition to binding and activating the PAK-like kinases Ste20 and Cla4, binds to the Ste11-Ste50 complex to bring activated Ste20/Cla4 to their substrate Ste11. Activated Ste11 and its HOG pathway-specific substrate, Pbs2, are brought together by Sho1; the Ste11-Ste50 complex binds to the cytoplasmic domain of Sho1, to which Pbs2 also binds. Thus, Cdc42, Ste50, and Sho1 act as adaptor proteins that control the flow of the osmostress signal from Ste20/Cla4 to Ste11, then to Pbs2.
Figures





References
-
- Brewster JL, de Valoir T, Dwyer ND, Winter E, Gustin MC (1993) An osmosensing signal transduction pathway in yeast. Science 259: 1760–1763 - PubMed
-
- Cvrckova F, De Virgilio C, Manser E, Pringle JR, Nasmyth K (1995) Ste20-like protein kinases are required for normal localization of cell growth and for cytokinesis in budding yeast. Genes Dev 9: 1817–1830 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

