Interleukin-2 signals during priming are required for secondary expansion of CD8+ memory T cells
- PMID: 16778891
- PMCID: PMC2776073
- DOI: 10.1038/nature04790
Interleukin-2 signals during priming are required for secondary expansion of CD8+ memory T cells
Abstract
Although interleukin-2 (IL-2) was initially characterized as the primary T-cell growth factor following in vitro activation, less is known about its role in shaping T-cell responses to acute infections in vivo. The use of IL-2- or IL-2-receptor-deficient mice is problematic owing to their early development of autoimmunity, attributable to the central role of IL-2 in the generation, maintenance and function of CD4+CD25+ regulatory T cells. To bypass these inherent difficulties, we have studied the effect of IL-2 on T-cell responses to acute infections by adopting a mixed chimaera strategy in which T cells lacking the high-affinity IL-2 receptor could be studied in an otherwise healthy mouse containing a full complement of regulatory T cells. Here we show that although IL-2 signalling to pathogen-specific CD8+ T cells affects the number of developing effector and memory cells very little, it is required for the generation of robust secondary responses. This is not due to an altered T-cell-receptor repertoire development or selection, and does not reflect an acute requirement for IL-2 during secondary activation and expansion. Rather, we demonstrate a previously unappreciated role for IL-2 during primary infection in programming the development of CD8+ memory T cells capable of full secondary expansion. These results have important implications for the development of vaccination or immunotherapeutic strategies aimed at boosting memory T-cell function.
Conflict of interest statement
Figures
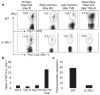
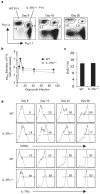


References
-
- Smith KA. Interleukin-2: inception, impact, and implications. Science. 1988;240:1169–1176. - PubMed
-
- Sadlack B, et al. Ulcerative colitis-like disease in mice with a disrupted interleukin-2 gene. Cell. 1993;75:253–261. - PubMed
-
- Schorle H, Holtschke T, Hunig T, Schimpl A, Horak I. Development and function of T cells in mice rendered interleukin-2 deficient by gene targeting. Nature. 1991;352:621–624. - PubMed
-
- Suzuki H, et al. Deregulated T cell activation and autoimmunity in mice lacking interleukin-2 receptor β. Science. 1995;268:1472–1476. - PubMed
-
- Willerford DM, et al. Interleukin-2 receptor α chain regulates the size and content of the peripheral lymphoid compartment. Immunity. 1995;3:521–530. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

