Direct binding of Cbl to Tyr568 and Tyr936 of the stem cell factor receptor/c-Kit is required for ligand-induced ubiquitination, internalization and degradation
- PMID: 16780420
- PMCID: PMC1570160
- DOI: 10.1042/BJ20060464
Direct binding of Cbl to Tyr568 and Tyr936 of the stem cell factor receptor/c-Kit is required for ligand-induced ubiquitination, internalization and degradation
Abstract
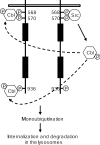
The ubiquitin E3 ligase Cbl has been shown to negatively regulate tyrosine kinase receptors, including the stem cell factor receptor/c-Kit. Impaired recruitment of Cbl to c-Kit results in a deregulated positive signalling that eventually can contribute to carcinogenesis. Here, we present results showing that Cbl is activated by the SFKs (Src family kinases) and recruited to c-Kit in order to trigger receptor ubiquitination. We demonstrate that phosphorylated Tyr568 and Tyr936 in c-Kit are involved in direct binding and activation of Cbl and that binding of the TKB domain (tyrosine kinase binding domain) of Cbl to c-Kit is specified by the presence of an isoleucine or leucine residue in position +3 to the phosphorylated tyrosine residue on c-Kit. Apart from the direct association between Cbl and c-Kit, we show that phosphorylation of Cbl by SFK members is required for activation of Cbl to occur. Moreover, we demonstrate that Cbl mediates monoubiquitination of c-Kit and that the receptor is subsequently targeted for lysosomal degradation. Taken together, our findings reveal novel insights into the mechanisms by which Cbl negatively regulates c-Kit-mediated signalling.
Figures






References
-
- Lennartsson J., Jelacic T., Linnekin D., Shivakrupa R. Normal and oncogenic forms of the receptor tyrosine kinase kit. Stem Cells. 2005;23:16–43. - PubMed
-
- Lennartsson J., Voytyuk O., Heiss E., Sundberg C., Sun J., Rönnstrand L. c-Kit signal transduction and involvement in cancer. Cancer Ther. 2005;3:5–28.
-
- Thien C. B., Langdon W. Y. Cbl: many adaptations to regulate protein tyrosine kinases. Nat. Rev. Mol. Cell Biol. 2001;2:294–307. - PubMed
-
- Lill N. L., Douillard P., Awwad R. A., Ota S., Lupher M. L., Jr, Miyake S., Meissner-Lula N., Hsu V. W., Band H. The evolutionarily conserved N-terminal region of Cbl is sufficient to enhance down-regulation of the epidermal growth factor receptor. J. Biol. Chem. 2000;275:367–377. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

