Long-lived effector/central memory T-cell responses to severe acute respiratory syndrome coronavirus (SARS-CoV) S antigen in recovered SARS patients
- PMID: 16781892
- PMCID: PMC7106132
- DOI: 10.1016/j.clim.2006.05.002
Long-lived effector/central memory T-cell responses to severe acute respiratory syndrome coronavirus (SARS-CoV) S antigen in recovered SARS patients
Abstract
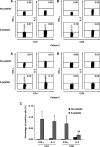
The role of cell-mediated immunity in human SARS-CoV infection is still not well understood. In this study, we found that memory T-cell responses against the spike (S) protein were persistent for more than 1 year after SARS-CoV infection by detecting the production of IFN-gamma using ELISA and ELISpot assays. Flow cytometric analysis showed that both CD4(+) and CD8(+) T cells were involved in cellular responses against SARS-CoV infection. Interestingly, most of SARS-CoV S-specific memory CD4(+) T cells were central memory cells expressing CD45RO(+) CCR7(+) CD62L(-). However, the majority of memory CD8(+) T cells revealed effector memory phenotype expressing CD45RO(-) CCR7(-) CD62L(-). Thus, our study provides the evidence that SARS-CoV infection in humans can induce cellular immune response that is persistent for a long period of time. These data may have an important implication in the possibility of designing effective vaccine against SARS-CoV infection, specifically in defining T-cell populations that are implicated in protective immunity.
Figures




References
-
- Drosten C., Günther S., Preiser W., Werf S., Brodt H.R., Becker S., Rabenau H., Panning M., Kolesnikova L., Fouchier R.A.M., Berger A., Burguiere A.M., Cinatl J., Eickmann M., Escriou N., Grywna K., Kramme S., Manuguerra J.C., Müller S., Rickerts V., Stürmer M., Vieth S., Klenk H.D., Osterhaus A.D.M.E., Schmitz H., Doerr H.W. Identification of a novel coronavirus in patients with severe acute respiratory syndrome. N. Engl. J. Med. 2003;348(20):1967–1976. - PubMed
-
- Peiris J.S.M., Yuen K.Y., Osterhaus A.D.M.E., Stöhr K. The severe acute respiratory syndrome. N. Engl. J. Med. 2003;349(25):2431–2441. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

