Transient loss of voltage control of Ca2+ release in the presence of maurocalcine in skeletal muscle
- PMID: 16782801
- PMCID: PMC1557560
- DOI: 10.1529/biophysj.105.078089
Transient loss of voltage control of Ca2+ release in the presence of maurocalcine in skeletal muscle
Abstract
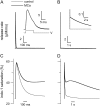
In skeletal muscle, sarcoplasmic reticulum (SR) calcium release is controlled by the plasma membrane voltage through interactions between the voltage-sensing dihydropyridine receptor (DHPr) and the ryanodine receptor (RYr) calcium release channel. Maurocalcine (MCa), a scorpion toxin peptide presenting some homology with a segment of a cytoplasmic loop of the DHPr, has been previously shown to strongly affect the activity of the isolated RYr. We injected MCa into mouse skeletal muscle fibers and measured intracellular calcium under voltage-clamp conditions. Voltage-activated calcium transients exhibited similar properties in control and in MCa-injected fibers during the depolarizing pulses, and the voltage dependence of calcium release was similar under the two conditions. However, MCa was responsible for a pronounced sustained phase of Ca(2+) elevation that proceeded for seconds following membrane repolarization, with no concurrent alteration of the membrane current. The magnitude of the underlying uncontrolled extra phase of Ca(2+) release correlated well with the peak calcium release during the pulse. Results suggest that MCa binds to RYr that open on membrane depolarization and that this interaction specifically alters the process of repolarization-induced closure of the channels.
Figures

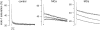




References
-
- Tanabe, T., K. G. Beam, B. A. Adams, T. Niidome, and S. Numa. 1990. Regions of the skeletal muscle dihydropyridine receptor critical for excitation-contraction coupling. Nature. 346:567–569. - PubMed
-
- Lu, X., L. Xu, and G. Meissner. 1994. Activation of the skeletal muscle calcium release channel by a cytoplasmic loop of the dihydropyridine receptor. J. Biol. Chem. 269:6511–6516. - PubMed
-
- Nakai, J., T. Tanabe, T. Konno, B. Adams, and K. G. Beam. 1998. Localization in the II–III loop of the dihydropyridine receptor of a sequence critical for excitation-contraction coupling. J. Biol. Chem. 273:24983–24986. - PubMed
-
- El-Hayek, R., B. Antoniu, J. Wang, S. L. Hamilton, and N. Ikemoto. 1995. Identification of calcium release-triggering and blocking regions of the II–III loop of the skeletal muscle dihydropyridine receptor. J. Biol. Chem. 270:22116–22118. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

