Defective mitochondrial gene expression results in reactive oxygen species-mediated inhibition of respiration and reduction of yeast life span
- PMID: 16782871
- PMCID: PMC1489155
- DOI: 10.1128/MCB.02360-05
Defective mitochondrial gene expression results in reactive oxygen species-mediated inhibition of respiration and reduction of yeast life span
Abstract
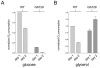
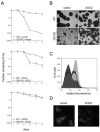
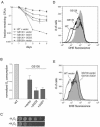
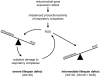
Mitochondrial dysfunction causes numerous human diseases and is widely believed to be involved in aging. However, mechanisms through which compromised mitochondrial gene expression elicits the reported variety of cellular defects remain unclear. The amino-terminal domain (ATD) of yeast mitochondrial RNA polymerase is required to couple transcription to translation during expression of mitochondrial DNA-encoded oxidative phosphorylation subunits. Here we report that several ATD mutants exhibit reduced chronological life span. The most severe of these (harboring the rpo41-R129D mutation) displays imbalanced mitochondrial translation, conditional inactivation of respiration, elevated production of reactive oxygen species (ROS), and increased oxidative stress. Reduction of ROS, via overexpression of superoxide dismutase (SOD1 or SOD2 product), not only greatly extends the life span of this mutant but also increases its ability to respire. Another ATD mutant with similarly reduced respiration (rpo41-D152A/D154A) accumulates only intermediate levels of ROS and has a less severe life span defect that is not rescued by SOD. Altogether, our results provide compelling evidence for the "vicious cycle" of mitochondrial ROS production and lead us to propose that the amount of ROS generated depends on the precise nature of the mitochondrial gene expression defect and initiates a downward spiral of oxidative stress only if a critical threshold is crossed.
Figures




References
-
- Balaban, R. S., S. Nemoto, and T. Finkel. 2005. Mitochondria, oxidants, and aging. Cell 120:483-495. - PubMed
-
- Bandy, B., and A. J. Davison. 1990. Mitochondrial mutations may increase oxidative stress: implications for carcinogenesis and aging? Free Radic. Biol. Med. 8:523-539. - PubMed
-
- Barros, M. H., B. Bandy, E. B. Tahara, and A. J. Kowaltowski. 2004. Higher respiratory activity decreases mitochondrial reactive oxygen release and increases life span in Saccharomyces cerevisiae. J. Biol. Chem. 279:49883-49888. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
