MondoA-Mlx heterodimers are candidate sensors of cellular energy status: mitochondrial localization and direct regulation of glycolysis
- PMID: 16782875
- PMCID: PMC1489152
- DOI: 10.1128/MCB.00657-05
MondoA-Mlx heterodimers are candidate sensors of cellular energy status: mitochondrial localization and direct regulation of glycolysis
Abstract
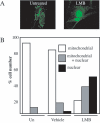
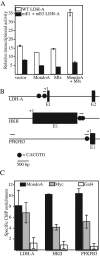
Transcription factors can be sequestered at specific organelles and translocate to the nucleus in response to changes in organellar homeostasis. MondoA is a basic helix-loop-helix leucine zipper transcriptional activator similar to Myc in function. However, unlike Myc, MondoA and its binding partner Mlx localize to the cytoplasm, suggesting tight regulation of their nuclear function. We show here that endogenous MondoA and Mlx associate with mitochondria in primary skeletal muscle cells and erythroblast K562 cells. Interaction between MondoA and the mitochondria is salt and protease sensitive, demonstrating that it associates with the outer mitochondrial membrane by binding a protein partner. Further, endogenous MondoA shuttles between the mitochondria and the nucleus, suggesting that it communicates between these two organelles. When nuclear, MondoA activates transcription of a broad spectrum of metabolic genes, including those for the glycolytic enzymes lactate dehydrogenase A, hexokinase II, and 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase 3. Regulation of these three targets is mediated by direct interaction with CACGTG sites in their promoters. Consistent with its regulation of glycolytic targets, MondoA is both necessary and sufficient for glycolysis. We propose that MondoA communicates information about the intracellular energy state between the mitochondria and the nucleus, resulting in transcriptional activation of glycolytic target genes.
Figures

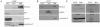

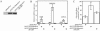

References
-
- Altomare, D. A., and J. R. Testa. 2005. Perturbations of the AKT signaling pathway in human cancer. Oncogene 24:7455-7464. - PubMed
-
- Atsumi, T., J. Chesney, C. Metz, L. Leng, S. Donnelly, Z. Makita, R. Mitchell, and R. Bucala. 2002. High expression of inducible 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase (iPFK-2; PFKFB3) in human cancers. Cancer Res. 62:5881-5887. - PubMed
-
- Baron, A., T. Migita, D. Tang, and M. Loda. 2004. Fatty acid synthase: a metabolic oncogene in prostate cancer? J. Cell. Biochem. 91:47-53. - PubMed
-
- Billin, A. N., and D. E. Ayer. 2006. The Mlx network: evidence for a parallel Max-like transcriptional network that regulates energy metabolism, p. 255-278. In R. N. Eisenman (ed.), The Myc/Max/Mad transcription factor network, vol. 302. Springer, Heidelberg, Germany. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
