Neuritic deposits of amyloid-beta peptide in a subpopulation of central nervous system-derived neuronal cells
- PMID: 16782885
- PMCID: PMC1489158
- DOI: 10.1128/MCB.00371-06
Neuritic deposits of amyloid-beta peptide in a subpopulation of central nervous system-derived neuronal cells
Abstract
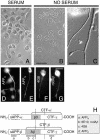
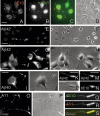
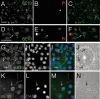
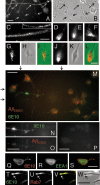
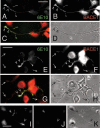
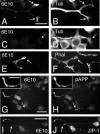
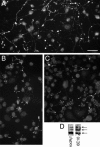
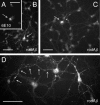
Our goal is to understand the pathogenesis of amyloid-beta (Abeta) deposition in the Alzheimer's disease (AD) brain. We established a cell culture system where central nervous system-derived neuronal cells (CAD cells) produce and accumulate within their processes large amounts of Abeta peptide, similar to what is believed to occur in brain neurons, in the initial phases of AD. Using this system, we show that accumulation of Abeta begins within neurites, prior to any detectable signs of neurodegeneration or abnormal vesicular transport. Neuritic accumulation of Abeta is restricted to a small population of neighboring cells that express normal levels of amyloid-beta precursor protein (APP) but show redistribution of BACE1 to the processes, where it colocalizes with Abeta and markers of late endosomes. Consistently, cells that accumulate Abeta appear in isolated islets, suggesting their clonal origin from a few cells that show a propensity to accumulate Abeta. These results suggest that Abeta accumulation is initiated in a small number of neurons by intracellular determinants that alter APP metabolism and lead to Abeta deposition and neurodegeneration. CAD cells appear to recapitulate the biochemical processes leading to Abeta deposition, thus providing an experimental in vitro system for studying the molecular pathobiology of AD.
Figures



References
-
- Arboleda, G., C. Waters, and R. M. Gibson. 2005. Metabolic activity: a novel indicator of neuronal survival in the murine dopaminergic cell line CAD. J. Mol. Neurosci 27:65-77. - PubMed
-
- Aston-Jones, G., and J. D. Cohen. 2005. An integrative theory of locus coeruleus-norepinephrine function: adaptive gain and optimal performance. Annu. Rev. Neurosci. 28:403-450. - PubMed
-
- Bard, F., R. Barbour, C. Cannon, R. Carretto, M. Fox, D. Games, T. Guido, K. Hoenow, K. Hu, K. Johnson-Wood, K. Khan, D. Kholodenko, C. Lee, M. Lee, R. Motter, M. Nguyen, A. Reed, D. Schenk, P. Tang, N. Vasquez, P. Seubert, and T. Yednock. 2003. Epitope and isotype specificities of antibodies to beta-amyloid peptide for protection against Alzheimer's disease-like neuropathology. Proc. Natl. Acad. Sci. USA 100:2023-2028. - PMC - PubMed
-
- Billings, L. M., S. Oddo, K. N. Green, J. L. McGaugh, and F. M. Laferla. 2005. Intraneuronal Aβ causes the onset of early Alzheimer's disease-related cognitive deficits in transgenic mice. Neuron 45:675-688. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
