Sphingosine-1-phosphate phosphohydrolase regulates endoplasmic reticulum-to-golgi trafficking of ceramide
- PMID: 16782891
- PMCID: PMC1489178
- DOI: 10.1128/MCB.02107-05
Sphingosine-1-phosphate phosphohydrolase regulates endoplasmic reticulum-to-golgi trafficking of ceramide
Abstract
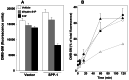
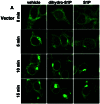
Previous studies demonstrated that sphingosine-1-phosphate (S1P) phosphohydrolase 1 (SPP-1), which is located mainly in the endoplasmic reticulum (ER), regulates sphingolipid metabolism and apoptosis (H. Le Stunff et al., J. Cell Biol. 158:1039-1049, 2002). We show here that the treatment of SPP-1-overexpressing cells with S1P, but not with dihydro-S1P, increased all ceramide species, particularly the long-chain ceramides. This was not due to inhibition of ceramide metabolism to sphingomyelin or monohexosylceramides but rather to the inhibition of ER-to-Golgi trafficking, determined with the fluorescent ceramide analog N-(4,4-difluoro-5,7-dimethyl-4-bora-3a,4a-diaza-s-indacene-3-pentanoyl)-d-erythro-sphingosine (DMB-Cer). Fumonisin B1, an inhibitor of ceramide synthase, prevented S1P-induced elevation of all ceramide species and corrected the defect in ER transport of DMB-Cer, readily allowing its detection in the Golgi. In contrast, ceramide accumulation had no effect on either the trafficking or the metabolism of 6-([N-(7-nitrobenzo-2-oxa-1,3-diazol-4-yl)amino]hexanoyl)-sphingosine, which rapidly labels the Golgi even at 4 degrees C. Protein trafficking from the ER to the Golgi, determined with vesicular stomatitis virus ts045 G protein fused to green fluorescent protein, was also inhibited in SPP-1-overexpressing cells in the presence of S1P but not in the presence of dihydro-S1P. Our results suggest that SPP-1 regulates ceramide levels in the ER and thus influences the anterograde membrane transport of both ceramide and proteins from the ER to the Golgi apparatus.
Figures
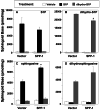
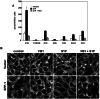
 ) for 24 h as described in Materials and Methods. Lipids were extracted and phosphorylated (A and B) and free sphingoid bases (C and D) were analyzed by ESI-MS/MS. The data are averages of triplicate determinations and are expressed as pmol lipid per mg of protein.
) for 24 h as described in Materials and Methods. Lipids were extracted and phosphorylated (A and B) and free sphingoid bases (C and D) were analyzed by ESI-MS/MS. The data are averages of triplicate determinations and are expressed as pmol lipid per mg of protein.  , P < 0.05 compared to vector.
, P < 0.05 compared to vector.
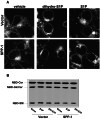
 , P < 0.05 compared to vector. (Inset) Cell-associated phospholipids from duplicate cultures after 24 h of treatment with [32P]S1P or [32P]dihydro-S1P (DH-S1P) were extracted and analyzed by TLC. Bands are labeled based on comigration with authentic unlabeled standards. LcbP, sphingoid base phosphates. A representative autoradiograph of a TLC plate is shown.
, P < 0.05 compared to vector. (Inset) Cell-associated phospholipids from duplicate cultures after 24 h of treatment with [32P]S1P or [32P]dihydro-S1P (DH-S1P) were extracted and analyzed by TLC. Bands are labeled based on comigration with authentic unlabeled standards. LcbP, sphingoid base phosphates. A representative autoradiograph of a TLC plate is shown.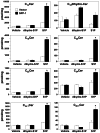
 , P < 0.05 compared to vector.
, P < 0.05 compared to vector.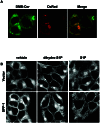



Similar articles
-
Genetic evidence for ATP-dependent endoplasmic reticulum-to-Golgi apparatus trafficking of ceramide for sphingomyelin synthesis in Chinese hamster ovary cells.J Cell Biol. 1999 Feb 22;144(4):673-85. doi: 10.1083/jcb.144.4.673. J Cell Biol. 1999. PMID: 10037789 Free PMC article.
-
A novel inhibitor of ceramide trafficking from the endoplasmic reticulum to the site of sphingomyelin synthesis.J Biol Chem. 2001 Nov 23;276(47):43994-4002. doi: 10.1074/jbc.M104884200. Epub 2001 Sep 6. J Biol Chem. 2001. PMID: 11546801
-
Sphingosine-1-phosphate phosphohydrolase in regulation of sphingolipid metabolism and apoptosis.J Cell Biol. 2002 Sep 16;158(6):1039-49. doi: 10.1083/jcb.200203123. Epub 2002 Sep 16. J Cell Biol. 2002. PMID: 12235122 Free PMC article.
-
CERT-mediated trafficking of ceramide.Biochim Biophys Acta. 2009 Jul;1791(7):684-91. doi: 10.1016/j.bbalip.2009.01.006. Epub 2009 Jan 22. Biochim Biophys Acta. 2009. PMID: 19416656 Review.
-
Molecular mechanisms and regulation of ceramide transport.Biochim Biophys Acta. 2005 Jun 1;1734(3):220-34. doi: 10.1016/j.bbalip.2005.04.001. Biochim Biophys Acta. 2005. PMID: 15907394 Review.
Cited by
-
Vitamin C Stimulates Epidermal Ceramide Production by Regulating Its Metabolic Enzymes.Biomol Ther (Seoul). 2015 Nov;23(6):525-30. doi: 10.4062/biomolther.2015.044. Epub 2015 Nov 1. Biomol Ther (Seoul). 2015. PMID: 26535077 Free PMC article.
-
Sphingolipids: key regulators of apoptosis and pivotal players in cancer drug resistance.Int J Mol Sci. 2014 Mar 12;15(3):4356-92. doi: 10.3390/ijms15034356. Int J Mol Sci. 2014. PMID: 24625663 Free PMC article. Review.
-
Shaping the landscape: metabolic regulation of S1P gradients.Biochim Biophys Acta. 2013 Jan;1831(1):193-202. doi: 10.1016/j.bbalip.2012.06.007. Epub 2012 Jun 23. Biochim Biophys Acta. 2013. PMID: 22735358 Free PMC article. Review.
-
Nuclear Translocation of SGPP-1 and Decrease of SGPL-1 Activity Contribute to Sphingolipid Rheostat Regulation of Inflammatory Dendritic Cells.Mediators Inflamm. 2017;2017:5187368. doi: 10.1155/2017/5187368. Epub 2017 Dec 11. Mediators Inflamm. 2017. PMID: 29375197 Free PMC article.
-
Sphingosine-1-phosphate phosphatase 1 regulates keratinocyte differentiation and epidermal homeostasis.J Biol Chem. 2013 Jun 21;288(25):18381-91. doi: 10.1074/jbc.M113.478420. Epub 2013 May 1. J Biol Chem. 2013. PMID: 23637227 Free PMC article.
References
-
- Baron, C. L., and V. Malhotra. 2002. Role of diacylglycerol in PKD recruitment to the TGN and protein transport to the plasma membrane. Science 295:325-328. - PubMed
-
- Chardin, P., and F. McCormick. 1999. Brefeldin A: the advantage of being uncompetitive. Cell 97:153-155. - PubMed
-
- Chen, C. S., A. G. Rosenwald, and R. E. Pagano. 1995. Ceramide as a modulator of endocytosis. J. Biol. Chem. 270:13291-13307. - PubMed
-
- Chigorno, V., C. Giannotta, E. Ottico, M. Sciannamblo, J. Mikulak, A. Prinetti, and S. Sonnino. 2005. Sphingolipid uptake by cultured cells: complex aggregates of cell sphingolipids with serum proteins and lipoproteins are rapidly catabolized. J. Biol. Chem. 280:2668-2675. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous
