Regulated activating Thr172 phosphorylation of cyclin-dependent kinase 4(CDK4): its relationship with cyclins and CDK "inhibitors"
- PMID: 16782892
- PMCID: PMC1489149
- DOI: 10.1128/MCB.02006-05
Regulated activating Thr172 phosphorylation of cyclin-dependent kinase 4(CDK4): its relationship with cyclins and CDK "inhibitors"
Abstract
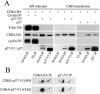
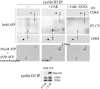
Cyclin-dependent kinase 4 (CDK4) is a master integrator of mitogenic and antimitogenic extracellular signals. It is also crucial for many oncogenic transformation processes. Various molecular features of CDK4 activation remain poorly known or debated, including the regulation of its association with D-type cyclins, its activating Thr172 phosphorylation, and the roles of Cip/Kip CDK "inhibitors" in these processes. Thr172 phosphorylation of CDK4 was reinvestigated using two-dimensional gel electrophoresis in various experimental systems, including human fibroblasts, canine thyroid epithelial cells stimulated by thyrotropin, and transfected mammalian and insect cells. Thr172 phosphorylation of CDK4 depended on prior D-type cyclin binding, but Thr172 phosphorylation was also found in p16-bound CDK4. Opposite effects of p27 on cyclin D3-CDK4 activity observed in different systems depended on its stoichiometry in this complex. Thr172-phosphorylated CDK4 was enriched in complexes containing p21 or p27, even at inhibitory levels of p27 that precluded CDK4 activity. Deletion of the p27 nuclear localization signal sequence relocalized cyclin D3-CDK4 in the cytoplasm but did not affect CDK4 phosphorylation. Within cyclin D3 complexes, T-loop phosphorylation of CDK4, but not of CDK6, was directly regulated, identifying it as a determining target for cell cycle control by extracellular factors. Collectively, these unexpected observations indicate that CDK4-activating kinase(s) should be reconsidered.
Figures







References
-
- Alt, J. R., A. B. Gladden, and J. A. Diehl. 2002. p21(Cip1) promotes cyclin D1 nuclear accumulation via direct inhibition of nuclear export. J. Biol. Chem. 277:8517-8523. - PubMed
-
- Aprelikova, O., Y. Xiong, and E. T. Liu. 1995. Both p16 and p21 families of cyclin-dependent kinase (CDK) inhibitors block the phosphorylation of cyclin-dependent kinases by the CDK-activating kinase. J. Biol. Chem. 270:18195-18197. - PubMed
-
- Baptist, M., F. Lamy, J. Gannon, T. Hunt, J. E. Dumont, and P. P. Roger. 1996. Expression and subcellular localization of CDK2 and cdc2 kinases and their common partner cyclin A in thyroid epithelial cells: comparison of cyclic AMP-dependent and -independent cell cycles. J. Cell. Physiol. 166:256-273. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
