Acetyl-keto-beta-boswellic acid inhibits cellular proliferation through a p21-dependent pathway in colon cancer cells
- PMID: 16783403
- PMCID: PMC1752013
- DOI: 10.1038/sj.bjp.0706817
Acetyl-keto-beta-boswellic acid inhibits cellular proliferation through a p21-dependent pathway in colon cancer cells
Abstract
1. Although there is increasing evidence showing that boswellic acid might be a potential anticancer agent, the mechanisms involved in its action are unclear. 2. In the present study, we showed that acetyl-keto-beta-boswellic acid (AKBA) inhibited cellular growth in several colon cancer cell lines. Cell cycle analysis by flow cytometry showed that cells were arrested at the G1 phase after AKBA treatment. 3. Further analysis showed that cyclin D1 and E, CDK 2 and 4 and phosphorylated Rb were decreased in AKBA-treated cells while p21 expression was increased. 4. The growth inhibitory effect of AKBA was dependent on p21 but not p53. HCT-116 p53(-/-) cells were sensitized to the apoptotic effect of AKBA, suggesting that p21 may have protected cells against apoptosis by inducing a G1 arrest.5. In conclusion, we have demonstrated that AKBA inhibited cellular growth in colon cancer cells. These findings may have implications to the use of boswellic acids as potential anticancer agents in colon cancer.
Figures



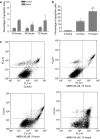


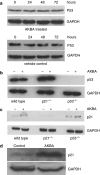

References
-
- CAPUTI M., RUSSO G., ESPOSITO V., MANCINI A., GIORDANO A. Role of cell-cycle regulators in lung cancer. J. Cell Physiol. 2005;205:319–327. - PubMed
-
- GARTEL A.L., RADHAKRISHNAN S.K. Lost in transcription: p21 repression, mechanisms, and consequences. Cancer Res. 2005;65:3980–3985. - PubMed
-
- GARTEL A.L., TYNER A.L. The role of the cyclin-dependent kinase inhibitor p21 in apoptosis. Mol. Cancer Ther. 2002;1:639–649. - PubMed
-
- GERHARDT H., SEIFERT F., BUVARI P., VOGELSANG H., REPGES R. Therapy of active Crohn disease with Boswellia serrata extract H 15] Z. Gastroenterol. 2001;39:11–17. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

