The role of urotensin II in cardiovascular and renal physiology and diseases
- PMID: 16783414
- PMCID: PMC1751922
- DOI: 10.1038/sj.bjp.0706800
The role of urotensin II in cardiovascular and renal physiology and diseases
Abstract
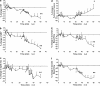
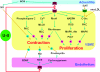
Urotensin II (U-II) is a cyclic neuropeptide that was first isolated from teleost fish some 35 years ago. Mammalian U-II is a powerful vasoconstrictor with a potency greater than that of endothelin-1.Nevertheless, unlike endothelin-1, which constricts all or nearly all vascular beds, the vasoactive effects of U-II are reported to be dependent both on the species and on the regional vascular bed examined. Typical regional variability occurs in the rat in which vasoconstriction to U-II is most robust in thoracic aorta proximal to the aortic arch and decreases gradually towards the distal peripheral arteries. As small peripheral arteries but not larger arteries such as the aorta play a major role in regulating peripheral resistance and consequent blood pressure as well as workload on the heart, doubts have been raised concerning the importance of this peptide in cardiovascular physiology. Moreover, an interaction between U-II and other endogenous vasoactive molecules may add a level of complexity to the vascular actions of U-II.On the other hand, recent experimental and clinical studies have revealed increased expression of U-II and urotensin receptor (UT receptor) in animals with experimentally induced myocardial infarction, heart failure, and in patients with hypertension, atherosclerosis, and diabetic nephropathy, which suggests a potential role for U-II in both cardiovascular and renal diseases. A series of peptidic and nonpeptidic UT receptor ligands have been shown to be effective in antagonizing the effects of U-II in the cardiorenal system. This article aims to review recent advances in our understanding of the physiology and pathophysiology of U-II with particular references to its role in cardiovascular health and disease.
Figures

Similar articles
-
Role of urotensin II in atherosclerotic cardiovascular diseases.Cardiovasc Revasc Med. 2008 Jul-Sep;9(3):166-78. doi: 10.1016/j.carrev.2008.02.001. Cardiovasc Revasc Med. 2008. PMID: 18606380 Review.
-
Emerging roles of urotensin-II in cardiovascular disease.Pharmacol Ther. 2004 Sep;103(3):223-43. doi: 10.1016/j.pharmthera.2004.07.004. Pharmacol Ther. 2004. PMID: 15464591 Review.
-
Urotensin II: the old kid in town.Trends Endocrinol Metab. 2004 May-Jun;15(4):175-82. doi: 10.1016/j.tem.2004.03.007. Trends Endocrinol Metab. 2004. PMID: 15109617 Review.
-
Urotensin II: its function in health and its role in disease.Cardiovasc Drugs Ther. 2005 Jan;19(1):65-75. doi: 10.1007/s10557-005-6899-x. Cardiovasc Drugs Ther. 2005. PMID: 15883758 Review.
-
Urotensin-II as a novel therapeutic target in the clinical management of cardiorenal disease.Curr Opin Investig Drugs. 2004 Mar;5(3):276-82. Curr Opin Investig Drugs. 2004. PMID: 15083593 Review.
Cited by
-
Can early myocardial infarction-related deaths be diagnosed using postmortem urotensin receptor expression levels?Forensic Sci Med Pathol. 2014 Sep;10(3):395-400. doi: 10.1007/s12024-014-9575-2. Epub 2014 Jun 18. Forensic Sci Med Pathol. 2014. PMID: 24935436
-
Urotensin II Protects Cardiomyocytes from Apoptosis Induced by Oxidative Stress through the CSE/H2S Pathway.Int J Mol Sci. 2015 Jun 3;16(6):12482-98. doi: 10.3390/ijms160612482. Int J Mol Sci. 2015. PMID: 26047336 Free PMC article.
-
The network map of urotensin-II mediated signaling pathway in physiological and pathological conditions.J Cell Commun Signal. 2022 Dec;16(4):601-608. doi: 10.1007/s12079-022-00672-4. Epub 2022 Feb 16. J Cell Commun Signal. 2022. PMID: 35174439 Free PMC article.
-
Role of urotensin II and its receptor in health and disease.J Anesth. 2007;21(3):378-89. doi: 10.1007/s00540-007-0524-z. Epub 2007 Aug 1. J Anesth. 2007. PMID: 17680191 Review.
-
Arrestins in apoptosis.Handb Exp Pharmacol. 2014;219:309-39. doi: 10.1007/978-3-642-41199-1_16. Handb Exp Pharmacol. 2014. PMID: 24292837 Free PMC article.
References
-
- AIYAR N., GUIDA B., AO Z., DISA J., NASELSKY D., BEHM D.J., SU J.L., KULL F.C., JR., DOUGLAS S.A. Differential levels of ‘urotensin-II-like' activity determined by radio-receptor and radioimmuno-assays. Peptides. 2004;25:1339–1347. - PubMed
-
- AMES R.S., SARAU H.M., CHAMBERS J.K., WILLETTE R.N., AIYAR N.V., ROMANIC A.M., LOUDEN C.S., FOLEY J.J., SAUERMELCH C.F., COATNEY R.W., AO Z., DISA J., HOLMES S.D., STADEL J.M., MARTIN J.D., LIU W.S., GLOVER G.I., WILSON S., MCNULTY D.E., ELLIS C.E., ELSHOURBAGY N.A., SHABON U., TRILL J.J., HAY D.W., OHLSTEIN E.H., BERGSMA D.J., DOUGLAS S.A. Human urotensin-II is a potent vasoconstrictor and agonist for the orphan receptor GPR14. Nature. 1999;401:282–286. - PubMed
-
- BALMENT R.J., SONG W., ASHTON N. Urotensin II: ancient hormone with new functions in vertebrate body fluid regulation. Ann. NY Acad. Sci. 2005;1040:66–73. - PubMed
-
- BEHM D.J., DOE C.P., JOHNS D.G., MANISCALCO K., STANKUS G.P., WIBBERLEY A., WILLETTE R.N., DOUGLAS S.A. Urotensin-II: a novel systemic hypertensive factor in the cat. Naunyn Schmiedebergs Arch. Pharmacol. 2004;369:274–280. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

