Physiological stimulation regulates the exocytic mode through calcium activation of protein kinase C in mouse chromaffin cells
- PMID: 16784416
- PMCID: PMC1570168
- DOI: 10.1042/BJ20060654
Physiological stimulation regulates the exocytic mode through calcium activation of protein kinase C in mouse chromaffin cells
Abstract
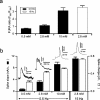
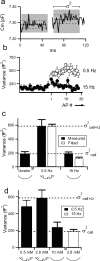
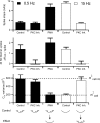
Adrenal medullary chromaffin cells release catecholamines and neuropeptides in an activity-dependent manner controlled by the sympathetic nervous system. Under basal sympathetic tone, catecholamines are preferentially secreted. During acute stress, increased sympathetic firing evokes release of both catecholamines as well as neuropeptides. Both signalling molecules are co-packaged in the same large dense core granules, thus release of neuropeptide transmitters must be regulated after granule fusion with the cell surface. Previous work has indicated this may be achieved through a size-exclusion mechanism whereby, under basal sympathetic firing, the catecholamines are selectively released through a restricted fusion pore, while less-soluble neuropeptides are left behind in the dense core. Only under the elevated firing experienced during the sympathetic stress response do the granules fully collapse to expel catecholamines and neuropeptides. However, mechanistic description and physiological regulation of this process remain to be determined. We employ electrochemical amperometry, fluid-phase dye uptake and electrophysiological capacitance noise analysis to probe the fusion intermediate in mouse chromaffin cells under physiological electrical stimulation. We show that basal firing rates result in the selective release of catecholamines through an Omega-form 'kiss and run' fusion event characterized by a narrow fusion pore. Increased firing raises calcium levels and activates protein kinase C, which then promotes fusion pore dilation until full granule collapse occurs. Our results demonstrate that the transition between 'kiss and run' and 'full collapse' exocytosis serves a vital physiological regulation in neuroendocrine chromaffin cells and help effect a proper acute stress response.
Figures


Similar articles
-
Activity-dependent differential transmitter release in mouse adrenal chromaffin cells.J Neurosci. 2005 Aug 10;25(32):7324-32. doi: 10.1523/JNEUROSCI.2042-05.2005. J Neurosci. 2005. PMID: 16093382 Free PMC article.
-
Cortical F-actin, the exocytic mode, and neuropeptide release in mouse chromaffin cells is regulated by myristoylated alanine-rich C-kinase substrate and myosin II.Mol Biol Cell. 2009 Jul;20(13):3142-54. doi: 10.1091/mbc.e09-03-0197. Epub 2009 May 6. Mol Biol Cell. 2009. Retraction in: Mol Biol Cell. 2013 Apr;24(8):1251. PMID: 19420137 Free PMC article. Retracted.
-
An activity-dependent increased role for L-type calcium channels in exocytosis is regulated by adrenergic signaling in chromaffin cells.Neuroscience. 2006 Dec 1;143(2):445-59. doi: 10.1016/j.neuroscience.2006.08.001. Epub 2006 Sep 8. Neuroscience. 2006. PMID: 16962713
-
Dynamin and myosin regulate differential exocytosis from mouse adrenal chromaffin cells.Cell Mol Neurobiol. 2010 Nov;30(8):1351-7. doi: 10.1007/s10571-010-9591-z. Cell Mol Neurobiol. 2010. PMID: 21061163 Free PMC article. Review.
-
Forty years of the adrenal chromaffin cell through ISCCB meetings around the world.Pflugers Arch. 2023 Jun;475(6):667-690. doi: 10.1007/s00424-023-02793-0. Epub 2023 Mar 8. Pflugers Arch. 2023. PMID: 36884064 Free PMC article. Review.
Cited by
-
Sustained Exocytosis after Action Potential-Like Stimulation at Low Frequencies in Mouse Chromaffin Cells Depends on a Dynamin-Dependent Fast Endocytotic Process.Front Cell Neurosci. 2016 Jul 26;10:184. doi: 10.3389/fncel.2016.00184. eCollection 2016. Front Cell Neurosci. 2016. PMID: 27507935 Free PMC article.
-
Increased catecholamine secretion from single adrenal chromaffin cells in DOCA-salt hypertension is associated with potassium channel dysfunction.ACS Chem Neurosci. 2013 Oct 16;4(10):1404-13. doi: 10.1021/cn400115v. Epub 2013 Aug 30. ACS Chem Neurosci. 2013. PMID: 23937098 Free PMC article.
-
Kiss-and-Run Is a Significant Contributor to Synaptic Exocytosis and Endocytosis in Photoreceptors.Front Cell Neurosci. 2017 Sep 20;11:286. doi: 10.3389/fncel.2017.00286. eCollection 2017. Front Cell Neurosci. 2017. PMID: 28979188 Free PMC article.
-
Phorbolester-activated Munc13-1 and ubMunc13-2 exert opposing effects on dense-core vesicle secretion.Elife. 2022 Oct 10;11:e79433. doi: 10.7554/eLife.79433. Elife. 2022. PMID: 36214779 Free PMC article.
-
Concentration of stimulant regulates initial exocytotic molecular plasticity at single cells.Chem Sci. 2022 Jan 20;13(6):1815-1822. doi: 10.1039/d1sc05278k. eCollection 2022 Feb 9. Chem Sci. 2022. PMID: 35282618 Free PMC article.
References
-
- Winkler H., Westhead E. The molecular organization of adrenal chromaffin granules. Neuroscience. 1980;5:1803–1823. - PubMed
-
- Takiyyuddin M. A., Cervenka J. H., Sullivan P. A., Pandian M. R., Parmer R. J., Barbosa J. A., O'Connor D. T. Is physiologic sympathoadrenal catecholamine release exocytotic in humans? Circulation. 1990;81:185–195. - PubMed
-
- Giampaolo B., Angelica M., Antonio S. Chromogranin ‘A’ in normal subjects, essential hypertensives and adrenalectomized patients. Clin. Endocrinol. 2002;57:41–50. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

