NMR spectra of oligosaccharides at ultra-high field (900 MHz) have better resolution than expected due to favourable molecular tumbling
- PMID: 16784734
- PMCID: PMC1828614
- DOI: 10.1016/j.carres.2006.05.017
NMR spectra of oligosaccharides at ultra-high field (900 MHz) have better resolution than expected due to favourable molecular tumbling
Abstract
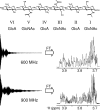
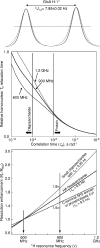
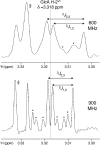
Nuclear magnetic resonance (NMR) remains the most promising technique for acquiring atomic-resolution information in complex carbohydrates. Significant obstacles to the acquisition of such data are the poor chemical-shift dispersion and artifacts resultant from their degenerate chemical structures. The recent development of ultra-high-field NMR (at 900 MHz and beyond) gives new potential to overcome these problems, as we demonstrate on a hexasaccharide of the highly repetitive glycosaminoglycan hyaluronan. At 900 MHz, the expected increase in spectral dispersion due to higher resonance frequencies and reduction in strong coupling-associated distortions are observed. In addition, the fortuitous molecular tumbling rate of oligosaccharides results in longer T2-values that further significantly enhances resolution, an effect not available to proteins. Combined, the resolution enhancement can be as much as twofold relative to 600 MHz, allowing all 1H-resonances in the hexasaccharide to be unambiguously assigned using standard natural-abundance experiments. The use of ultra-high-field spectrometers is clearly advantageous and promises a new and exciting era in carbohydrate structural biology.
Figures
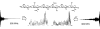

Similar articles
-
Use of 15N-NMR to resolve molecular details in isotopically-enriched carbohydrates: sequence-specific observations in hyaluronan oligomers up to decasaccharides.Glycobiology. 2004 Nov;14(11):999-1009. doi: 10.1093/glycob/cwh117. Epub 2004 Jun 23. Glycobiology. 2004. PMID: 15215231
-
920 MHz ultra-high field NMR approaches to structural glycobiology.Biochim Biophys Acta. 2008 Mar;1780(3):619-25. doi: 10.1016/j.bbagen.2007.11.014. Epub 2007 Dec 5. Biochim Biophys Acta. 2008. PMID: 18157953
-
Complete assignment of hyaluronan oligosaccharides up to hexasaccharides.Carbohydr Res. 2006 Dec 11;341(17):2803-15. doi: 10.1016/j.carres.2006.09.023. Epub 2006 Oct 4. Carbohydr Res. 2006. PMID: 17056022
-
Enabling methodology for the end functionalization of glycosaminoglycan oligosaccharides.Mol Biosyst. 2008 Jun;4(6):481-95. doi: 10.1039/b801666f. Epub 2008 Apr 17. Mol Biosyst. 2008. PMID: 18493641 Review.
-
Applications of 2D NMR spectroscopy to carbohydrates.Basic Life Sci. 1990;56:17-25. doi: 10.1007/978-1-4684-5868-8_3. Basic Life Sci. 1990. PMID: 2078170 Review. No abstract available.
Cited by
-
A 3D-structural model of unsulfated chondroitin from high-field NMR: 4-sulfation has little effect on backbone conformation.Carbohydr Res. 2010 Jan 26;345(2):291-302. doi: 10.1016/j.carres.2009.11.013. Epub 2009 Nov 23. Carbohydr Res. 2010. PMID: 20022001 Free PMC article.
-
Primary Structure of Glycans by NMR Spectroscopy.Chem Rev. 2023 Feb 8;123(3):1040-1102. doi: 10.1021/acs.chemrev.2c00580. Epub 2023 Jan 9. Chem Rev. 2023. PMID: 36622423 Free PMC article. Review.
-
A refined model for the TSG-6 link module in complex with hyaluronan: use of defined oligosaccharides to probe structure and function.J Biol Chem. 2014 Feb 28;289(9):5619-34. doi: 10.1074/jbc.M113.542357. Epub 2014 Jan 8. J Biol Chem. 2014. PMID: 24403066 Free PMC article.
-
Is N-acetyl-D-glucosamine a rigid 4C1 chair?Glycobiology. 2011 Dec;21(12):1651-62. doi: 10.1093/glycob/cwr101. Epub 2011 Aug 1. Glycobiology. 2011. PMID: 21807769 Free PMC article.
-
NMR of Macromolecular Assemblies and Machines at 1 GHz and Beyond: New Transformative Opportunities for Molecular Structural Biology.Methods Mol Biol. 2018;1688:1-35. doi: 10.1007/978-1-4939-7386-6_1. Methods Mol Biol. 2018. PMID: 29151202 Free PMC article. Review.
References
-
- Almond A., DeAngelis P.L., Blundell C.D. J. Am. Chem. Soc. 2005;127:1086–1087. - PubMed
-
- Homans S.W. Biochem. Soc. Trans. 1998;26:551–560. - PubMed
-
- Cowman M.K., Matsuoka S. Carbohydr. Res. 2005;340:791–809. - PubMed
-
- Martin-Pastor M., Bush C.A. J. Biomol. NMR. 2001;19:125–139. - PubMed
-
- Milton M.J., Harris R., Probert M.A., Field R.A., Homans S.W. Glycobiology. 1998;8:147–153. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

