Mechanics of actomyosin bonds in different nucleotide states are tuned to muscle contraction
- PMID: 16785439
- PMCID: PMC1502541
- DOI: 10.1073/pnas.0601255103
Mechanics of actomyosin bonds in different nucleotide states are tuned to muscle contraction
Abstract
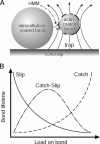
Muscle contraction and many other cell movements are driven by cyclic interactions between actin filaments and the motor enzyme myosin. Conformational changes in the actin-myosin binding interface occur in concert with the binding of ATP, binding to actin, and loss of hydrolytic by-products, but the effects of these conformational changes on the strength of the actomyosin bond are unknown. The force-dependent kinetics of the actomyosin bond may be particularly important at high loads, where myosin may detach from actin before achieving its full power stroke. Here we show that over a physiological range of rapidly applied loads, actomyosin behaves as a "catch" bond, characterized by increasing lifetimes with increasing loads up to a maximum at approximately 6 pN. Surprisingly, we found that the myosin-ADP bond is possessed of longer lifetimes under load than rigor bonds, although the load at which bond lifetime is maximal remains unchanged. We also found that actomyosin bond lifetime is ultimately dependent not only on load, but loading history as well. These data suggest a complex relationship between the rate of actomyosin dissociation and muscle force and shortening velocity. The 6-pN load for maximum bond lifetime is near the force generated by a single myosin molecule during isometric contraction. This raises the possibility that all catch bonds between load-bearing molecules are "mechanokinetically" tuned to their physiological environment.
Conflict of interest statement
Conflict of interest statement: No conflicts declared.
Figures



References
-
- Rayment I., Rypniewski W. R., Schmidt-Base K., Smith R., Tomchick D. R., Benning M. M., Winkelmann D. A., Wesenberg G., Holden H. M. Science. 1993;261:50–58. - PubMed
-
- Rayment I., Holden H. M., Whittaker M., Yohn C. B., Lorenz M., Holmes K. C., Milligan R. A. Science. 1993;261:58–65. - PubMed
-
- Warshaw D. M., Guilford W. H., Freyzon Y., Krementsova E., Palmiter K. A., Tyska M. J., Baker J. E., Trybus K. M. J. Biol. Chem. 2000;275:37167–37172. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

