The B30.2 domain of pyrin, the familial Mediterranean fever protein, interacts directly with caspase-1 to modulate IL-1beta production
- PMID: 16785446
- PMCID: PMC1479864
- DOI: 10.1073/pnas.0602081103
The B30.2 domain of pyrin, the familial Mediterranean fever protein, interacts directly with caspase-1 to modulate IL-1beta production
Abstract
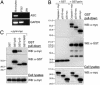
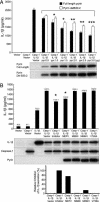
Familial Mediterranean fever (FMF) is a recessively inherited autoinflammatory disorder with high carrier frequencies in the Middle East. Pyrin, the protein mutated in FMF, regulates caspase-1 activation and consequently IL-1beta production through cognate interaction of its N-terminal PYRIN motif with the ASC adaptor protein. However, the preponderance of mutations reside in pyrin's C-terminal B30.2 domain. Here we demonstrate direct interaction of this domain with caspase-1. In lysates from cells not expressing ASC, reciprocal GST pull-downs demonstrated the interaction of pyrin with the p20 and p10 catalytic subunits of caspase-1. Coimmunoprecipitations of pyrin and caspase-1 from THP-1 human monocytic cells were consistent with the interaction of endogenous proteins. The C-terminal B30.2 domain of pyrin is necessary and sufficient for the interaction, and binding was reduced by FMF-associated B30.2 mutations. Full-length pyrin attenuated IL-1beta production in cells transfected with a caspase-1/IL-1beta construct, an effect diminished by FMF-associated B30.2 mutations and in B30.2 deletion mutants. Modeling of the crystal structure of caspase-1 with the deduced structure of the pyrin B30.2 domain corroborated both the interaction and the importance of M694V and M680I pyrin mutations. Consistent with a net inhibitory effect of pyrin on IL-1beta activation, small interfering RNA (siRNA)-mediated pyrin knockdown in THP-1 cells augmented IL-1beta production in response to bacterial LPS. Moreover, the IL-1 receptor antagonist anakinra suppressed acute-phase proteins in a patient with FMF and amyloidosis. Our data support a direct, ASC-independent effect of pyrin on IL-1beta activation and suggest heightened IL-1 responsiveness as one factor selecting for pyrin mutations.
Conflict of interest statement
Conflict of interest statement: No conflicts declared.
Figures
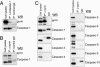




References
-
- Galon J., Aksentijevich I., McDermott M. F., O’Shea J. J., Kastner D. L. Curr. Opin. Immunol. 2000;12:479–486. - PubMed
-
- Stojanov S., Kastner D. L. Curr. Opin. Rheumatol. 2005;17:586–599. - PubMed
-
- Kastner D. L., Aksentijevich I. Arthritis and Allied Conditions. In: Koopman W. J., Moreland L. W., editors. Philadelphia: Lippincott Williams and Wilkins; 2005. pp. 1411–1461.
-
- International FMF Consortium. Cell. Vol. 90. 1997. pp. 797–807. - PubMed
-
- French FMF Consortium. Nat. Genet. 1997;17:25–31. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

