Increased level and longevity of protective immune responses induced by DNA vaccine expressing the HIV-1 Env glycoprotein when combined with IL-21 and IL-15 gene delivery
- PMID: 16785513
- PMCID: PMC2504862
- DOI: 10.4049/jimmunol.177.1.177
Increased level and longevity of protective immune responses induced by DNA vaccine expressing the HIV-1 Env glycoprotein when combined with IL-21 and IL-15 gene delivery
Abstract
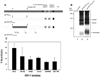
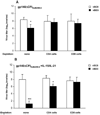
We investigated the ability of a plasmid-derived IL-21 delivered alone or in combination with the IL-15 gene to regulate immune responses to the HIV-1 envelope (Env) glycoprotein induced by DNA vaccination. Mice were injected with the gp140DeltaCFI(HXB2/89.6) vector expressing a modified Env glycoprotein with C-terminal mutations intended to mimic a fusion intermediate, in which the most divergent region encoding the variable V1, V2, and V3 domains of CXCR4-tropic HxB2 virus was replaced with the dual-tropic 89.6 viral strain. Using a recombinant vaccinia virus expressing 89.6 Env glycoprotein (vBD3) in a mouse challenge model, we observed that IL-21 plasmid produced sustained resistance to viral transmission when injected 5 days after DNA vaccination. Moreover, IL-21 in a synergistic manner with IL-15 expression vector augmented the vaccine-induced recall responses to the vBD3 challenge compared with those elicited by immunization in the presence of either cytokine alone. The synergistic combination of IL-21 and IL-15 plasmids promoted expansion of CD8+CD127+ memory T cell pools specific for a subdominant HLA-A2-restricted Env(121-129) epitope (KLTPLCVTL). Our results also show that coimmunization with IL-21 and IL-15 plasmid combination resulted in enhanced CD8+ T cell function that was partially independent of CD4+ T cell help in mediating protection against vBD3 challenge. Furthermore, the use of IL-21 and IL-15 genes was able to increase Ab-dependent cellular cytotoxicity and complement-dependent lysis of Env-expressing target cells through augmentation of Env-specific IgG Ab levels. These data indicate that the plasmid-delivered IL-21 and IL-15 can increase the magnitude of the response to DNA vaccines.
Conflict of interest statement
The authors have no financial conflict of interest.
Figures







Similar articles
-
Improving recombinant MVA immune responses: potentiation of the immune responses to HIV-1 with MVA and DNA vectors expressing Env and the cytokines IL-12 and IFN-gamma.Virus Res. 2006 Mar;116(1-2):11-20. doi: 10.1016/j.virusres.2005.08.008. Epub 2005 Oct 7. Virus Res. 2006. PMID: 16214252
-
Induction of HIV immunity in the genital tract after intranasal delivery of a MVA vector: enhanced immunogenicity after DNA prime-modified vaccinia virus Ankara boost immunization schedule.J Immunol. 2004 May 15;172(10):6209-20. doi: 10.4049/jimmunol.172.10.6209. J Immunol. 2004. PMID: 15128809
-
Studies of the neutralizing activity and avidity of anti-human immunodeficiency virus type 1 Env antibody elicited by DNA priming and protein boosting.J Virol. 1998 Nov;72(11):9092-100. doi: 10.1128/JVI.72.11.9092-9100.1998. J Virol. 1998. PMID: 9765454 Free PMC article.
-
Mucosal immunization of lactating female rhesus monkeys with a transmitted/founder HIV-1 envelope induces strong Env-specific IgA antibody responses in breast milk.J Virol. 2013 Jun;87(12):6986-99. doi: 10.1128/JVI.00528-13. Epub 2013 Apr 17. J Virol. 2013. PMID: 23596289 Free PMC article.
-
Preclinical and clinical development of a multi-envelope, DNA-virus-protein (D-V-P) HIV-1 vaccine.Int Rev Immunol. 2009;28(1):49-68. doi: 10.1080/08830180802495605. Int Rev Immunol. 2009. PMID: 19241253 Free PMC article. Review.
Cited by
-
Interleukin-21 augments the efficacy of T-cell therapy by eliciting concurrent cellular and humoral responses.Cancer Res. 2008 Jun 1;68(11):4431-41. doi: 10.1158/0008-5472.CAN-07-5530. Cancer Res. 2008. PMID: 18519706 Free PMC article.
-
Directing T-Cell Immune Responses for Cancer Vaccination and Immunotherapy.Vaccines (Basel). 2021 Nov 25;9(12):1392. doi: 10.3390/vaccines9121392. Vaccines (Basel). 2021. PMID: 34960140 Free PMC article. Review.
-
Expression of interleukin-15 and interleukin-15Rα in monocytes of HIV type 1-infected patients with different courses of disease progression.AIDS Res Hum Retroviruses. 2012 Jul;28(7):693-701. doi: 10.1089/AID.2010.0317. Epub 2011 Sep 23. AIDS Res Hum Retroviruses. 2012. PMID: 21902580 Free PMC article.
-
Improvement in efficacy of DNA vaccine encoding HIV-1 Vif by LIGHT gene adjuvant.Viral Immunol. 2013 Feb;26(1):68-74. doi: 10.1089/vim.2012.0073. Epub 2013 Jan 18. Viral Immunol. 2013. PMID: 23330678 Free PMC article.
-
Elevation of Tim-3 and PD-1 expression on T cells appears early in HIV infection, and differential Tim-3 and PD-1 expression patterns can be induced by common γ -chain cytokines.Biomed Res Int. 2015;2015:916936. doi: 10.1155/2015/916936. Epub 2015 Jan 22. Biomed Res Int. 2015. PMID: 25685816 Free PMC article.
References
-
- Kiszka I, Kmieciak D, Gzyl J, Naito T, Bolesta E, Sieron A, Singh SP, Srinivasan A, Trinchieri G, Kaneko Y, Kozbor D. Effect of the V3 loop deletion of envelope glycoprotein on cellular responses and protection against challenge with recombinant vaccinia virus expressing gp160 of primary human immunodeficiency virus type 1 isolates. J. Virol. 2002;76:4222–4232. - PMC - PubMed
-
- Donnelly JJ, Wahren B, Liu MA. DNA vaccines: progress and challenges. J. Immunol. 2005;175:633–639. - PubMed
-
- Hanke T, McMichael AJ, Samuel RV, Powell LA, McLoughlin L, Crome SJ, Edlin A. Lack of toxicity and persistence in the mouse associated with administration of candidate DNA- and modified vaccinia virus Ankara (MVA)-based HIV vaccines for Kenya. Vaccine. 2002;21:108–114. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

