Conserved nontypeable Haemophilus influenzae-derived TLR2-binding lipopeptides synergize with IFN-beta to increase cytokine production by resident murine and human alveolar macrophages
- PMID: 16785566
- PMCID: PMC2373263
- DOI: 10.4049/jimmunol.177.1.673
Conserved nontypeable Haemophilus influenzae-derived TLR2-binding lipopeptides synergize with IFN-beta to increase cytokine production by resident murine and human alveolar macrophages
Abstract
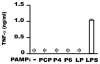
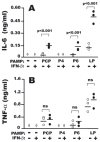
Nontypeable Haemophilus influenzae (NTHi) is strongly associated with exacerbations of chronic obstructive pulmonary disease, which often coincide with viral respiratory infections. TLR2 contributes importantly to innate immunity to NTHi, but whether this pathway is affected by simultaneous antiviral responses is unknown. To analyze potential interactions, resident murine and human alveolar macrophages (AMphi) were exposed, in the presence or absence of the appropriate rIFN-beta, to synthetic lipopeptides corresponding to the triacylated N-terminal fragments of three outer membrane proteins (OMP) (PCP, P4, and P6) that are highly conserved among different NTHi strains. Synthetic OMP elicited strong release of IL-6, the principal inducer of airway mucin genes, and induced CCL5 and CXCL10 from murine AMphi only when IFN-beta was also present. Surprisingly, combined stimulation by OMPs and IFN-beta also markedly enhanced TNF-alpha release by murine AMphi. Stimulation with PCP plus IFN-beta induced IFN-regulatory factor 1 expression and sustained STAT1 activation, but did not alter the activation of MAPKs or NF-kappaB. AMphi derived from STAT1-deficient mice did not demonstrate increased production of TNF-alpha in response to PCP plus IFN-beta. Analysis of wild-type and STAT1-deficient AMphi using real-time PCR showed that increased TNF-alpha production depended on transcriptional up-regulation, but not on mRNA stabilization. The synergistic effect of synthetic OMP and IFN-beta was conserved between murine AMphi and human AMphi for IL-6, but not for TNF-alpha. Thus, IFN-beta, which is produced by virally infected respiratory epithelial cells, converts normally innocuous NTHi OMP into potent inflammatory stimulants, but does so via different mechanisms in mice and humans.
Conflict of interest statement
Disclosures
The authors have no financial conflict of interest.
Figures
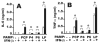
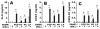
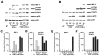
Similar articles
-
Outer membrane protein P6 of nontypeable Haemophilus influenzae is a potent and selective inducer of human macrophage proinflammatory cytokines.Infect Immun. 2005 May;73(5):2728-35. doi: 10.1128/IAI.73.5.2728-2735.2005. Infect Immun. 2005. PMID: 15845475 Free PMC article.
-
Specific engagement of TLR4 or TLR3 does not lead to IFN-beta-mediated innate signal amplification and STAT1 phosphorylation in resident murine alveolar macrophages.J Immunol. 2004 Jul 15;173(2):1033-42. doi: 10.4049/jimmunol.173.2.1033. J Immunol. 2004. PMID: 15240691 Free PMC article.
-
The MyD88-dependent, but not the MyD88-independent, pathway of TLR4 signaling is important in clearing nontypeable haemophilus influenzae from the mouse lung.J Immunol. 2005 Nov 1;175(9):6042-9. doi: 10.4049/jimmunol.175.9.6042. J Immunol. 2005. PMID: 16237099
-
Type I interferon induced by DNA of nontypeable Haemophilus influenza modulates inflammatory cytokine profile to promote susceptibility to this bacterium.Int Immunopharmacol. 2019 Sep;74:105710. doi: 10.1016/j.intimp.2019.105710. Epub 2019 Jun 27. Int Immunopharmacol. 2019. PMID: 31255879
-
Impaired innate immune alveolar macrophage response and the predilection for COPD exacerbations.Thorax. 2014 Sep;69(9):811-8. doi: 10.1136/thoraxjnl-2013-203669. Epub 2014 Mar 31. Thorax. 2014. PMID: 24686454
Cited by
-
Lung CD8+ T cells in COPD have increased expression of bacterial TLRs.Respir Res. 2013 Feb 1;14(1):13. doi: 10.1186/1465-9921-14-13. Respir Res. 2013. PMID: 23374856 Free PMC article. Clinical Trial.
-
A novel TLR2 agonist from Bordetella pertussis is a potent adjuvant that promotes protective immunity with an acellular pertussis vaccine.Mucosal Immunol. 2015 May;8(3):607-17. doi: 10.1038/mi.2014.93. Epub 2014 Oct 15. Mucosal Immunol. 2015. PMID: 25315966
-
Effects of cigarette smoke on Toll-like receptor (TLR) activation of chronic obstructive pulmonary disease (COPD) macrophages.Clin Exp Immunol. 2014 Jun;176(3):461-72. doi: 10.1111/cei.12289. Clin Exp Immunol. 2014. PMID: 24528166 Free PMC article.
-
Fusobacterium necrophorum Promotes Apoptosis and Inflammatory Cytokine Production Through the Activation of NF-κB and Death Receptor Signaling Pathways.Front Cell Infect Microbiol. 2022 Jun 14;12:827750. doi: 10.3389/fcimb.2022.827750. eCollection 2022. Front Cell Infect Microbiol. 2022. PMID: 35774408 Free PMC article.
-
TLR2 mediates gap junctional intercellular communication through connexin-43 in intestinal epithelial barrier injury.J Biol Chem. 2009 Aug 14;284(33):22332-22343. doi: 10.1074/jbc.M901619200. Epub 2009 Jun 15. J Biol Chem. 2009. PMID: 19528242 Free PMC article.
References
-
- Kuklinska D, Kilian M. Relative proportions of Haemophilus species in the throat of healthy children and adults. Eur J Clin Microbiol. 1984;3:249–252. - PubMed
-
- Wilson R. Evidence of bacterial infection in acute exacerbations of chronic bronchitis. Semin Respir Infect. 2000;15:208–215. - PubMed
-
- Sethi S, Evans N, Grant BJ, Murphy TF. New strains of bacteria and exacerbations of chronic obstructive pulmonary disease. N Engl J Med. 2002;347:465– 471. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

