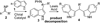Asymmetric Friedel-crafts reaction of indoles with imines by an organic catalyst
- PMID: 16787078
- PMCID: PMC3161403
- DOI: 10.1021/ja062700v
Asymmetric Friedel-crafts reaction of indoles with imines by an organic catalyst
Abstract
In this communication, we report an asymmetric Friedel-Crafts reaction of indoles with imines catalyzed by a bifunctional cinchona alkaloid catalyst. This is the first efficient organocatalytic asymmetric Friedel-Crafts reaction of indoles with imines. This reaction is operationally simple and, unprecedentedly, affords high enantioselectivity for a wide range of indoles and both aryl and alkyl imines. This establishes a direct, convergent, and versatile approach to optically active 3-indolyl methanamines, a structural motif embedded in numerous indole alkaloids and synthetic indole derivatives.
Figures
References
-
- Atta-ur-Rahman, Basha A. Indole Alkaloids. Harwood academic; 1998.
- Kutney JP. The synthesis of Indole Alkaloids. In: ApSimon J, editor. The Total Synthesis of Natural Products. Vol. 3. A Wiley-Interscience; 1977. p. pp 273.
- Faulkner DJ. Nat. Prod. Rep. 2002;1 - PubMed
- Amat M, Llor N, Bosch J, Solans X. Tetrahedron. 1997;53:719.
-
-
For recent reviews of catalytic asymmetric Friedel-Crafts reactions see: Bandini M, Melloni A, Umani-Ronchi A. Angew. Chem., Int. Ed. 2004;43:550. Jørgensen KA. Synthesis. 2003:1117.
-
-
-
For select examples of catalytic asymmetric Michael additions of indoles by chiral metal complexes see: Evans DA, Scheidt KA, Fandrick KR, Lam HW, Wu J. J. Am. Chem. Soc. 2003;125:10780. Evans DA, Fandrick KR, Song H-J. J. Am. Chem. Soc. 2005;127:8942. Palomo C, Oiarbide M, Kardak BG, Garcia JM, Linden A. J. Am. Chem. Soc. 2005;127:4154.
-
-
-
For Michael additions of indoles catalyzed by chiral organic catalysts see: Austin JF, MacMillan DWC. J. Am. Chem. Soc. 2002;124:1172. Huang Y, Walji AM, Larsen CH, MacMillan DWC. J. Am. Chem. Soc. 2005;127:15051. Austin JF, Kim S-G, Sinz CJ, Xiao W-J, MacMillan DWC. Proc. Natl. Acad. Sci USA. 2004;101:5482. Herrera RP, Sgarzani V, Bernardi L, Ricci A. Angew. Chem. Int. Ed. 2005;44:6576.
-
-
-
For catalytic asymmetric additions of indoles to ethyl 3,3,3-trifluoropyruvate see: Zhuang W, Gathergood N, Hazell RG, Jørgensen KA. J. Org. Chem. 2001;66:1009. Török B, Abid M, London G, Esquibel J, Török MS, Mhadgut C, Yan P, Prakash GKS. Angew. Chem. Int. Ed. 2005;44:3086.
-
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources