A mechanism of cell death involving an adenylyl cyclase/PKA signaling pathway is induced by the Cry1Ab toxin of Bacillus thuringiensis
- PMID: 16788061
- PMCID: PMC1502550
- DOI: 10.1073/pnas.0604017103
A mechanism of cell death involving an adenylyl cyclase/PKA signaling pathway is induced by the Cry1Ab toxin of Bacillus thuringiensis
Abstract
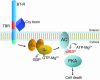
Many pathogenic organisms and their toxins target host cell receptors, the consequence of which is altered signaling events that lead to aberrant activity or cell death. A significant body of literature describes various molecular and cellular aspects of toxins associated with bacterial invasion, colonization, and host cell disruption. However, there is little information on the molecular and cellular mechanisms associated with the insecticidal action of Bacillus thuringiensis (Bt) Cry toxins. Recently, we reported that the Cry1Ab toxin produced by Bt kills insect cells by activating a Mg(2+)-dependent cytotoxic event upon binding of the toxin to its receptor BT-R(1). Here we show that binding of Cry toxin to BT-R(1) provokes cell death by activating a previously undescribed signaling pathway involving stimulation of G protein (G(alphas)) and adenylyl cyclase, increased cAMP levels, and activation of protein kinase A. Induction of the adenylyl cyclase/protein kinase A pathway is manifested by sequential cytological changes that include membrane blebbing, appearance of ghost nuclei, cell swelling, and lysis. The discovery of a toxin-induced cell death pathway specifically linked to BT-R(1) in insect cells should provide insights into how insects evolve resistance to Bt and into the development of new, safer insecticides.
Conflict of interest statement
Conflict of interest statement: No conflicts declared.
Figures
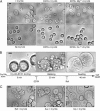
References
-
- Ashkenazi A., Dixit V. M. Science. 1998;281:1305–1308. - PubMed
-
- Lai E. C. Development (Cambridge, U.K.) 2004;131:965–973. - PubMed
-
- Gilman A. G. Annu. Rev. Biochem. 1987;56:615–649. - PubMed
-
- Juliano R. L. Annu. Rev. Pharmacol. Toxicol. 2002;42:283–323. - PubMed
-
- Finlay B. B., Cossart P. Science. 1997;276:718–725. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

