Anopheles gambiae immune responses to human and rodent Plasmodium parasite species
- PMID: 16789837
- PMCID: PMC1475661
- DOI: 10.1371/journal.ppat.0020052
Anopheles gambiae immune responses to human and rodent Plasmodium parasite species
Abstract
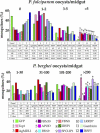
Transmission of malaria is dependent on the successful completion of the Plasmodium lifecycle in the Anopheles vector. Major obstacles are encountered in the midgut tissue, where most parasites are killed by the mosquito's immune system. In the present study, DNA microarray analyses have been used to compare Anopheles gambiae responses to invasion of the midgut epithelium by the ookinete stage of the human pathogen Plasmodium falciparum and the rodent experimental model pathogen P. berghei. Invasion by P. berghei had a more profound impact on the mosquito transcriptome, including a variety of functional gene classes, while P. falciparum elicited a broader immune response at the gene transcript level. Ingestion of human malaria-infected blood lacking invasive ookinetes also induced a variety of immune genes, including several anti-Plasmodium factors. Twelve selected genes were assessed for effect on infection with both parasite species and bacteria using RNAi gene silencing assays, and seven of these genes were found to influence mosquito resistance to both parasite species. An MD2-like receptor, AgMDL1, and an immunolectin, FBN39, showed specificity in regulating only resistance to P. falciparum, while the antimicrobial peptide gambicin and a novel putative short secreted peptide, IRSP5, were more specific for defense against the rodent parasite P. berghei. While all the genes that affected Plasmodium development also influenced mosquito resistance to bacterial infection, four of the antimicrobial genes had no effect on Plasmodium development. Our study shows that the impact of P. falciparum and P. berghei infection on A. gambiae biology at the gene transcript level is quite diverse, and the defense against the two Plasmodium species is mediated by antimicrobial factors with both universal and Plasmodium-species specific activities. Furthermore, our data indicate that the mosquito is capable of sensing infected blood constituents in the absence of invading ookinetes, thereby inducing anti-Plasmodium immune responses.
Conflict of interest statement
Figures



References
-
- Osta MA, Christophides GK, Vlachou D, Kafatos FC. Innate immunity in the malaria vector Anopheles gambiae: Comparative and functional genomics. J Exp Biol. 2004;207:2551–2563. - PubMed
-
- Osta MA, Christophides GK, Kafatos FC. Effects of mosquito genes on Plasmodium development. Science. 2004;303:2030–2032. - PubMed
-
- Kim W, Koo H, Richman AM, Seeley D, Vizioli J, et al. Ectopic expression of a cecropin transgene in the human malaria vector mosquito Anopheles gambiae (Diptera: Culicidae): Effects on susceptibility to Plasmodium . J Med Entomol. 2004;41:447–455. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

