CCR5 is essential for NK cell trafficking and host survival following Toxoplasma gondii infection
- PMID: 16789839
- PMCID: PMC1475660
- DOI: 10.1371/journal.ppat.0020049
CCR5 is essential for NK cell trafficking and host survival following Toxoplasma gondii infection
Abstract
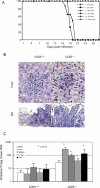
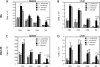
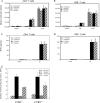
The host response to intracellular pathogens requires the coordinated action of both the innate and acquired immune systems. Chemokines play a critical role in the trafficking of immune cells and transitioning an innate immune response into an acquired response. We analyzed the host response of mice deficient in the chemokine receptor CCR5 following infection with the intracellular protozoan parasite Toxoplasma gondii. We found that CCR5 controls recruitment of natural killer (NK) cells into infected tissues. Without this influx of NK cells, tissues from CCR5-deficient (CCR5-/-) mice were less able to generate an inflammatory response, had decreased chemokine and interferon gamma production, and had higher parasite burden. As a result, CCR5-/- mice were more susceptible to infection with T. gondii but were less susceptible to the immune-mediated tissue injury seen in certain inbred strains. Adoptive transfer of CCR5+/+ NK cells into CCR5-/- mice restored their ability to survive lethal T. gondii infection and demonstrated that CCR5 is required for NK cell homing into infected liver and spleen. This study establishes CCR5 as a critical receptor guiding NK cell trafficking in host defense.
Conflict of interest statement
Figures



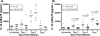
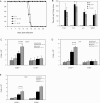
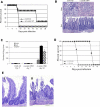

References
-
- Sher A, Denkers E, Gazzinelli R. Induction and regulation of host cell-mediated immunity by Toxoplasma gondii . Ciba Found Symp. 1995;195:95–104. - PubMed
-
- Bliss SK, Butcher BA, Denkers EY. Rapid recruitment of neutrophils containing prestored IL-12 during microbial infection. J Immunol. 2000;165:4515–4521. - PubMed
-
- Khan IA, Murphy PM, Casciotti L, Schwartzman JD, Collins J, et al. Mice lacking the chemokine receptor CCR1 show increased susceptibility to Toxoplasma gondii infection. J Immunol. 2001;166:1930–1937. - PubMed
-
- Suzuki Y, Orellana M, Schreiber R, Remington J. Interferon-gammon: The major mediator of resistance against Toxoplasma gondii . Science. 1988;22:516–518. - PubMed
-
- Suzuki Y, Remington J. Dual regulation of resistance against Toxoplasma gondii infection by Lyt-2+ and Lyt-1+, L3T4+ T cells in mice. J Immunol. 1988;140:3943–3946. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

