Early events in Mycobacterium tuberculosis infection in cynomolgus macaques
- PMID: 16790751
- PMCID: PMC1489679
- DOI: 10.1128/IAI.00064-06
Early events in Mycobacterium tuberculosis infection in cynomolgus macaques
Abstract
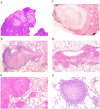
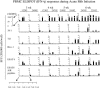
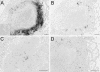
Little is known regarding the early events of infection of humans with Mycobacterium tuberculosis. The cynomolgus macaque is a useful model of tuberculosis, with strong similarities to human tuberculosis. In this study, eight cynomolgus macaques were infected bronchoscopically with low-dose M. tuberculosis; clinical, immunologic, microbiologic, and pathologic events were assessed 3 to 6 weeks postinfection. Gross pathological abnormalities were observed as early as 3 weeks, including Ghon complex formation by 5 weeks postinfection. Caseous granulomas were observed in the lung as early as 4 weeks postinfection. Only caseous granulomas were observed in the lungs at these early time points, reflecting a rigorous initial response. T-cell activation (CD29 and CD69) and chemokine receptor (CXCR3 and CCR5) expression appeared localized to different anatomic sites. Activation markers were increased on cells from airways and only at modest levels on cells in peripheral blood. The priming of mycobacterium-specific T cells, characterized by the production of gamma interferon occurred slowly, with responses seen only after 4 weeks of infection. These responses were observed from T lymphocytes in blood, airways, and hilar lymph node, with responses predominantly localized to the site of infection. From these studies, we conclude that immune responses to M. tuberculosis are relatively slow in the local and peripheral compartments and that necrosis occurs surprisingly quickly during granuloma formation.
Figures


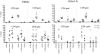

References
-
- Algood, H. M., P. L. Lin, D. Yankura, A. Jones, J. Chan, and J. L. Flynn. 2004. TNF influences chemokine expression of macrophages in vitro and that of CD11b+ cells in vivo during Mycobacterium tuberculosis infection. J. Immunol. 172:6846-6857. - PubMed
-
- Barry, S. M., M. C. Lipman, B. Bannister, M. A. Johnson, and G. Janossy. 2003. Purified protein derivative-activated type 1 cytokine-producing CD4+ T lymphocytes in the lung: a characteristic feature of active pulmonary and nonpulmonary tuberculosis. J. Infect. Dis. 187:243-250. - PubMed
-
- Capuano, S. V. I., D. A. Croix, S. Pawar, A. Zinovik, A. Myers, P. L. Lin, S. Bissel, C. Fuhrman, E. Klein, and J. L. Flynn. 2003. Experimental Mycobacterium tuberculosis infection of cynomolgus macaques closely resembles the various manifestations of human M. tuberculosis infection. Infect. Immun. 71:5831-5844. - PMC - PubMed
-
- Choi, Y. K., B. A. Fallert, M. A. Murphey-Corb, and T. A. Reinhart. 2003. Simian immunodeficiency virus dramatically alters expression of homeostatic chemokines and dendritic cell markers during infection in vivo. Blood 101:1684-1691. - PubMed
-
- Fallert, B. A., and T. A. Reinhart. 2002. Improved detection of simian immunodeficiency virus RNA by in situ hybridization in fixed tissue sections: combined effects of temperatures for tissue fixation and probe hybridization. J. Virol. Methods 99:23-32. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

