Previous or ongoing schistosome infections do not compromise the efficacy of the attenuated cercaria vaccine
- PMID: 16790771
- PMCID: PMC1489735
- DOI: 10.1128/IAI.01657-05
Previous or ongoing schistosome infections do not compromise the efficacy of the attenuated cercaria vaccine
Abstract
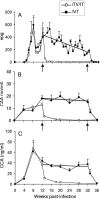
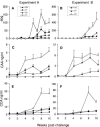
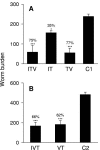
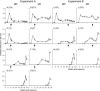
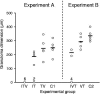
A current or previous schistosome infection might compromise the efficacy of a schistosome vaccine administered to humans. We have therefore investigated the influence of infection on vaccination, using the baboon as the model host and irradiated Schistosoma mansoni cercariae as the vaccine. Protection, determined from worm burdens in test and controls, was not diminished when vaccination was superimposed on a chronic infection, nor was it diminished when it followed a primary infection terminated by chemotherapy. Protection was also assessed indirectly based on fecal egg output and circulating antigen levels, as would be the case in human vaccine trials. In almost all instances, these methods overestimated protection, sometimes with discrepancies of >20%. The overwhelming immune response to egg deposition in infected animals made it difficult to discern a contribution from vaccination. Nevertheless, the well-documented immunomodulation of immune responses that follows egg deposition did not appear to impede the protective mechanisms elicited by vaccination with attenuated cercariae.
Figures
References
-
- Ashton, P. D., R. S. Curwen, and R. A. Wilson. 2001. Linking proteome and genome: how to identify parasite proteins. Trends Parasitol. 17:198-202. - PubMed
-
- Butterworth, A. E., P. R. Dalton, D. W. Dunne, M. Mugambi, J. H. Ouma, B. A. Richardson, T. K. Siongok, and R. F. Sturrock. 1984. Immunity after treatment of human schistosomiasis mansoni. I. Study design, pretreatment observations, and the results of treatment. Trans. R. Soc. Trop. Med. Hyg. 78:108-123. - PubMed
-
- Coulson, P. S. 1997. The radiation-attenuated vaccine against schistosomes in animal models: paradigm for a human vaccine? Adv. Parasitol. 39:271-336. - PubMed
-
- Curwen, R. S., P. D. Ashton, D. A. Johnston, and R. A. Wilson. 2004. The Schistosoma mansoni soluble proteome: a comparison across four life-cycle stages. Mol. Biochem. Parasitol. 138:57-66. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

