The Actinobacillus actinomycetemcomitans ribose binding protein RbsB interacts with cognate and heterologous autoinducer 2 signals
- PMID: 16790775
- PMCID: PMC1489740
- DOI: 10.1128/IAI.01741-05
The Actinobacillus actinomycetemcomitans ribose binding protein RbsB interacts with cognate and heterologous autoinducer 2 signals
Abstract
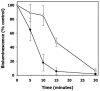
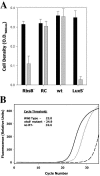
Autoinducer 2 (AI-2) produced by the oral pathogen Actinobacillus actinomycetemcomitans influences growth of the organism under iron limitation and regulates the expression of iron uptake genes. However, the cellular components that mediate the response of A. actinomycetemcomitans to AI-2 have not been fully characterized. Analysis of the complete genome sequence of A. actinomycetemcomitans (www.oralgen.lanl.gov) indicated that the RbsB protein was related to LuxP, the AI-2 receptor of Vibrio harveyi. To determine if RbsB interacts with AI-2, the bioluminescence of the reporter strain V. harveyi BB170 (sensor 1-, sensor 2+) was determined after stimulation with partially purified AI-2 from A. actinomycetemcomitans or conditioned medium from V. harveyi cultures in the presence and absence of purified six-His-tagged RbsB. RbsB efficiently inhibited V. harveyi bioluminescence induced by both A. actinomycetemcomitans AI-2 and V. harveyi AI-2 in a dose-dependent manner, suggesting that RbsB competes with LuxP for AI-2. Fifty percent inhibition occurred with approximately 0.3 nM RbsB for A. actinomycetemcomitans AI-2 and 15 nM RbsB for V. harveyi AI-2. RbsB-mediated inhibition of V. harveyi bioluminescence was reversed by the addition of 50 mM ribose, suggesting that A. actinomycetemcomitans AI-2 and ribose bind at the same site of RbsB. The RbsB/AI-2 complex was thermostable since A. actinomycetemcomitans AI-2 could not be recovered by heating. This was not due to heat inactivation of A. actinomycetemcomitans AI-2 since signal activity was unaffected by heating in the absence of RbsB. Furthermore, an isogenic A. actinomycetemcomitans mutant that was unable to express rbsB was deficient in depleting A. actinomycetemcomitans AI-2 from solution relative to the wild-type organism. Inactivation of rbsB also influenced the ability of the organism to grow under iron-limiting conditions. The mutant strain attained a cell density of approximately 30% that of the wild-type organism under iron limitation. In addition, real-time PCR showed that the expression of afuABC, encoding a major ferric ion transporter, was reduced by approximately eightfold in the rbsB mutant. This phenotype was similar to that of a LuxS-deficient mutant of A. actinomycetemcomitans that is unable to produce AI-2. Together, our results suggest that RbsB may play a role in the response of A. actinomycetemcomitans to AI-2.
Figures

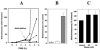

Similar articles
-
Differential interaction of Aggregatibacter (Actinobacillus) actinomycetemcomitans LsrB and RbsB proteins with autoinducer 2.J Bacteriol. 2007 Aug;189(15):5559-65. doi: 10.1128/JB.00387-07. Epub 2007 May 25. J Bacteriol. 2007. PMID: 17526716 Free PMC article.
-
Autoinducer 2 is required for biofilm growth of Aggregatibacter (Actinobacillus) actinomycetemcomitans.Infect Immun. 2007 Sep;75(9):4211-8. doi: 10.1128/IAI.00402-07. Epub 2007 Jun 25. Infect Immun. 2007. PMID: 17591788 Free PMC article.
-
Intra- and interspecies regulation of gene expression by Actinobacillus actinomycetemcomitans LuxS.Infect Immun. 2001 Dec;69(12):7625-34. doi: 10.1128/IAI.69.12.7625-7634.2001. Infect Immun. 2001. PMID: 11705942 Free PMC article.
-
luxS and arcB control aerobic growth of Actinobacillus actinomycetemcomitans under iron limitation.Infect Immun. 2003 Jan;71(1):298-308. doi: 10.1128/IAI.71.1.298-308.2003. Infect Immun. 2003. PMID: 12496179 Free PMC article.
-
Oxazaborolidine derivatives inducing autoinducer-2 signal transduction in Vibrio harveyi.Bioorg Med Chem. 2008 Feb 15;16(4):1596-604. doi: 10.1016/j.bmc.2007.11.032. Epub 2007 Nov 17. Bioorg Med Chem. 2008. PMID: 18053731 Review.
Cited by
-
D-Galactose as an autoinducer 2 inhibitor to control the biofilm formation of periodontopathogens.J Microbiol. 2016 Sep;54(9):632-637. doi: 10.1007/s12275-016-6345-8. Epub 2016 Aug 31. J Microbiol. 2016. PMID: 27572513
-
Construction of new cloning, lacZ reporter and scarless-markerless suicide vectors for genetic studies in Aggregatibacter actinomycetemcomitans.Plasmid. 2013 May;69(3):211-22. doi: 10.1016/j.plasmid.2013.01.002. Epub 2013 Jan 23. Plasmid. 2013. PMID: 23353051 Free PMC article.
-
The absence of luxS reduces the invasion of Avibacterium paragallinarum but is not essential for virulence.Front Vet Sci. 2024 Aug 27;11:1427966. doi: 10.3389/fvets.2024.1427966. eCollection 2024. Front Vet Sci. 2024. PMID: 39263678 Free PMC article.
-
Inhibition of Plasmid Conjugation in Escherichia coli by Targeting rbsB Gene Using CRISPRi System.Int J Mol Sci. 2023 Jun 24;24(13):10585. doi: 10.3390/ijms241310585. Int J Mol Sci. 2023. PMID: 37445761 Free PMC article.
-
Inhibitory effect of paeoniflorin on the LuxS/AI-2 quorum sensing system and the virulence of Glaesserella parasuis.J Vet Med Sci. 2025 May 15;87(5):565-574. doi: 10.1292/jvms.25-0008. Epub 2025 Mar 31. J Vet Med Sci. 2025. PMID: 40159223 Free PMC article.
References
-
- Bassler, B. L. 1999. How bacteria talk to each other: regulation of gene expression by quorum sensing. Curr. Opin. Microbiol. 2:582-587. - PubMed
-
- Bassler, B. L. 2002. Small talk: cell-to-cell communication in bacteria. Cell 109:421-424. - PubMed
-
- Bassler, B. L., M. Wright, and M. R. Silverman. 1994. Multiple signaling systems controlling expression of luminescence in Vibrio harveyi: sequence and function of genes encoding a second sensory pathway. Mol. Microbiol. 13:273-286. - PubMed
-
- Block, P. J., A. C. Fox, C. Yoran, and A. J. Kaltman. 1973. Actinobacillus actinomycetemcomitans endocarditis: report of a case and review of the literature. Am. J. Med. Sci. 276:387-392. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

