Burkholderia cenocepacia ZmpB is a broad-specificity zinc metalloprotease involved in virulence
- PMID: 16790782
- PMCID: PMC1489746
- DOI: 10.1128/IAI.00297-06
Burkholderia cenocepacia ZmpB is a broad-specificity zinc metalloprotease involved in virulence
Abstract
In previous studies we characterized the Burkholderia cenocepacia ZmpA zinc metalloprotease. In this study, we determined that B. cenocepacia has an additional metalloprotease, which we designated ZmpB. The zmpB gene is present in the same species as zmpA and was detected in B. cepacia, B. cenocepacia, B. stabilis, B. ambifaria, and B. pyrrocinia but was absent from B. multivorans, B. vietnamiensis, B. dolosa, and B. anthina. The zmpB gene was expressed, and ZmpB was purified from Escherichia coli by using the pPROEXHTa His(6) Tag expression system. ZmpB has a predicted preproenzyme structure typical of thermolysin-like proteases and is distantly related to Bacillus cereus bacillolysin. ZmpB was expressed as a 63-kDa preproenzyme precursor that was autocatalytically cleaved into mature ZmpB (35 kDa) and a 27-kDa prepropeptide. EDTA, 1,10-phenanthroline, and Zn(2+) cations inhibited ZmpB enzyme activity, indicating that it is a metalloprotease. ZmpB had proteolytic activity against alpha-1 proteinase inhibitor, alpha(2)-macrogobulin, type IV collagen, fibronectin, lactoferrin, transferrin, and immunoglobulins. B. cenocepacia zmpB and zmpA zmpB mutants had no proteolytic activity against casein and were less virulent in a rat agar bead chronic infection model, indicating that zmpB is involved in B. cenocepacia virulence. Expression of zmpB was regulated by both the CepIR and CciIR quorum-sensing systems.
Figures
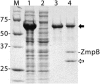

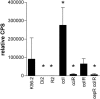

References
-
- Cash, H. A., D. E. Woods, B. McCullough, W. G. Johanson, Jr., and J. A. Bass. 1979. A rat model of chronic respiratory infection with Pseudomonas aeruginosa. Am. Rev. Respir. Dis. 119:453-459. - PubMed
-
- Chang, A. K., H. Y. Kim, J. E. Park, P. Acharya, I. S. Park, S. M. Yoon, H. J. You, K. S. Hahm, J. K. Park, and J. S. Lee. 2005. Vibrio vulnificus secretes a broad-specificity metalloprotease capable of interfering with blood homeostasis through prothrombin activation and fibrinolysis. J. Bacteriol. 187:6909-6916. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

