Blastocystis ratti induces contact-independent apoptosis, F-actin rearrangement, and barrier function disruption in IEC-6 cells
- PMID: 16790785
- PMCID: PMC1489721
- DOI: 10.1128/IAI.00328-06
Blastocystis ratti induces contact-independent apoptosis, F-actin rearrangement, and barrier function disruption in IEC-6 cells
Abstract
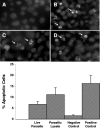
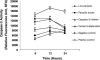
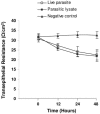
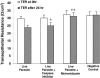
Blastocystis is an enteric protozoan purportedly associated with numerous clinical cases of diarrhea, flatulence, vomiting, and other gastrointestinal symptoms. Despite new knowledge of Blastocystis cell biology, genetic diversity, and epidemiology, its pathogenic potential remains controversial. Numerous clinical and epidemiological studies either implicate or exonerate the parasite as a cause of intestinal disease. Therefore, the aim of this study was to investigate the pathogenic potential of Blastocystis by studying the interactions of Blastocystis ratti WR1, an isolate of zoonotic potential, with a nontransformed rat intestinal epithelial cell line, IEC-6. Here, we report that B. ratti WR1 induces apoptosis in IEC-6 cells in a contact-independent manner. Furthermore, we found that B. ratti WR1 rearranges F-actin distribution, decreases transepithelial resistance, and increases epithelial permeability in IEC-6 cell monolayers. In addition, we found that the effects of B. ratti on transepithelial electrical resistance and epithelial permeability were significantly abrogated by treatment with metronidazole, an antiprotozoal drug. Our results suggest for the first time that Blastocystis-induced apoptosis in host cells and altered epithelial barrier function might play an important role in the pathogenesis of Blastocystis infections and that metronidazole has therapeutic potential in alleviating symptoms associated with Blastocystis.
Figures

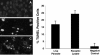


References
-
- Alexeieff, A. 1911. Sur la nature des formations dites kystes de Trichomonas intestinalis. C. R. Soc. Biol. 71:296-298.
-
- Ashford, R. W., and E. A. Atkinson. 1992. Epidemiology of Blastocystis hominis infection in Papua New Guinea: age-prevalence and associations with other parasites. Ann. Trop. Med. Parasitol. 86:129-136. - PubMed
-
- Bjarnason, I., A. MacPherson, and D. Hollander. 1995. Intestinal permeability: an overview. Gastroenterology 108:1566-1581. - PubMed
-
- Bojarski, C., K. Bendfeldt, A. H. Gitter, J. Mankertz, M. Fromm, S. Wagner, E. O. Riecken, and J. D. Schulzke. 2000. Apoptosis and intestinal barrier function. Ann. N. Y. Acad. Sci. 915:270-274. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

