Use of an Actinobacillus pleuropneumoniae multiple mutant as a vaccine that allows differentiation of vaccinated and infected animals
- PMID: 16790786
- PMCID: PMC1489739
- DOI: 10.1128/IAI.00133-06
Use of an Actinobacillus pleuropneumoniae multiple mutant as a vaccine that allows differentiation of vaccinated and infected animals
Abstract
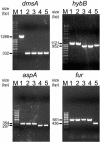
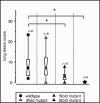
Vaccination against Actinobacillus pleuropneumoniae is hampered by the lack of vaccines inducing reliable cross-serotype protection. In contrast, pigs surviving natural infection are at least partially protected from clinical symptoms upon reinfection with any serotype. Thus, we set out to construct an attenuated A. pleuropneumoniae live vaccine allowing the differentiation of vaccinated from infected animals (the DIVA concept) by successively deleting virulence-associated genes. Based on an A. pleuropneumoniae serotype 2 prototype live negative marker vaccine (W. Tonpitak, N. Baltes, I. Hennig-Pauka, and G.-F. Gerlach, Infect. Immun. 70:7120-7125, 2002), genes encoding three enzymes involved in anaerobic respiration and the ferric uptake regulator Fur were deleted, resulting in a highly attenuated sixfold mutant; this mutant was still able to colonize the lower respiratory tract and induced a detectable immune response. Upon a single aerosol application, this mutant provided significant protection from clinical symptoms upon heterologous infection with an antigenically distinct A. pleuropneumoniae serotype 9 challenge strain and allowed the serological discrimination between infected and vaccinated groups.
Figures

Similar articles
-
Construction of an Actinobacillus pleuropneumoniae serotype 2 prototype live negative-marker vaccine.Infect Immun. 2002 Dec;70(12):7120-5. doi: 10.1128/IAI.70.12.7120-7125.2002. Infect Immun. 2002. PMID: 12438394 Free PMC article.
-
Deletion of the znuA virulence factor attenuates Actinobacillus pleuropneumoniae and confers protection against homologous or heterologous strain challenge.Vet Microbiol. 2014 Dec 5;174(3-4):531-539. doi: 10.1016/j.vetmic.2014.10.016. Epub 2014 Oct 24. Vet Microbiol. 2014. PMID: 25465668
-
Development of a DIVA subunit vaccine against Actinobacillus pleuropneumoniae infection.Vaccine. 2006 Nov 30;24(49-50):7226-37. doi: 10.1016/j.vaccine.2006.06.047. Epub 2006 Jul 7. Vaccine. 2006. PMID: 17027123
-
New trends in innovative vaccine development against Actinobacillus pleuropneumoniae.Vet Microbiol. 2018 Apr;217:66-75. doi: 10.1016/j.vetmic.2018.02.028. Epub 2018 Mar 6. Vet Microbiol. 2018. PMID: 29615259 Review.
-
Actinobacillus pleuropneumoniae vaccines: from bacterins to new insights into vaccination strategies.Anim Health Res Rev. 2008 Jun;9(1):25-45. doi: 10.1017/S1466252307001338. Epub 2008 Mar 17. Anim Health Res Rev. 2008. PMID: 18346296 Review.
Cited by
-
Experimental Actinobacillus pleuropneumoniae challenge in swine: comparison of computed tomographic and radiographic findings during disease.BMC Vet Res. 2012 Apr 30;8:47. doi: 10.1186/1746-6148-8-47. BMC Vet Res. 2012. PMID: 22546414 Free PMC article.
-
Immunization of pigs to prevent disease in humans: construction and protective efficacy of a Salmonella enterica serovar Typhimurium live negative-marker vaccine.Infect Immun. 2007 May;75(5):2476-83. doi: 10.1128/IAI.01908-06. Epub 2007 Feb 12. Infect Immun. 2007. PMID: 17296750 Free PMC article.
-
Analysis of the Actinobacillus pleuropneumoniae ArcA regulon identifies fumarate reductase as a determinant of virulence.Infect Immun. 2008 Jun;76(6):2284-95. doi: 10.1128/IAI.01540-07. Epub 2008 Mar 31. Infect Immun. 2008. PMID: 18378638 Free PMC article.
-
In vivo testing of novel vaccine prototypes against Actinobacillus pleuropneumoniae.Vet Res. 2018 Jan 9;49(1):4. doi: 10.1186/s13567-017-0502-x. Vet Res. 2018. PMID: 29316978 Free PMC article.
-
Immunization of pigs with Actinobacillus pleuropneumonia live attenuated (gene-deleted) vaccine HB04M intramuscularly or intranasally exhibits remarkably rapid protection against heterologous strain challenge.BMC Vet Res. 2025 Jul 8;21(1):450. doi: 10.1186/s12917-025-04895-6. BMC Vet Res. 2025. PMID: 40629392 Free PMC article.
References
-
- Baltes, N., I. Hennig-Pauka, and G.-F. Gerlach. 2002. Both transferrin binding proteins are virulence factors in Actinobacillus pleuropneumoniae serotype 7 infection. FEMS Microbiol. Lett. 209:283-287. - PubMed
-
- Baltes, N., S. Kyaw, I. Hennig-Pauka, and G.-F. Gerlach. 2004. Lack of influence of the anaerobic [NiFe] hydrogenase and L-1,2 propanediol oxidoreductase on the outcome of Actinobacillus pleuropneumoniae serotype 7 infection. Vet. Microbiol. 102:67-72. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

