Sialic acid-binding immunoglobulin-like lectin 7 mediates selective recognition of sialylated glycans expressed on Campylobacter jejuni lipooligosaccharides
- PMID: 16790787
- PMCID: PMC1489752
- DOI: 10.1128/IAI.02094-05
Sialic acid-binding immunoglobulin-like lectin 7 mediates selective recognition of sialylated glycans expressed on Campylobacter jejuni lipooligosaccharides
Abstract
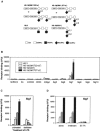
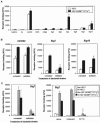
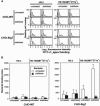
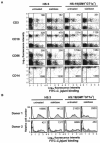
siglecs are a family of sialic-acid binding immunoglobulin-like lectins mostly expressed by cells of the immune system that have the potential to interact with sialylated glycans expressed not only on host cells but also on certain pathogens. Campylobacter jejuni is a common pathogen of humans that expresses surface lipooligosaccharides (LOS) that can be modified with ganglioside-like terminal structures in the core oligosaccharides. In this study, we examined the interaction of 10 siglecs with LOS purified from four different C. jejuni isolates expressing GM1-like, GD1a-like, GD3-like, and GT1a-like oligosaccharides. Of all siglecs examined, only Siglec-7 exhibited specific, sialic acid-dependent interactions with C. jejuni LOS in solid-phase binding assays. Binding was especially prominent with LOS from the HS:19(GM1(+) GT1a(+)) isolate, with weaker binding with LOS from the HS:19(GD3(+)) isolate. Binding of Siglec-7 was also observed with intact bacteria expressing these LOS structures. Specific binding of HS:19(GM1(+) GT1a(+)) bacteria was demonstrated with Siglec-7 expressed on transfected Chinese hamster ovary cells and with peripheral blood leukocytes, among which HS:19(GM1(+) GT1a(+)) bacteria bound selectively to both natural killer cells and monocytes which naturally express Siglec-7. These results raise the possibility that, in addition to their role in generating autoimmune antibody responses, C. jejuni LOS could interact with Siglec-7 expressed by leukocytes, modulate the host-pathogen interaction, and contribute to the clinical outcome and the development of secondary complications such as Guillain-Barré syndrome.
Figures

References
-
- Aspinall, G. O., C. M. Lynch, H. Pang, R. T. Shaver, and A. P. Moran. 1995. Chemical structures of the core region of Campylobacter jejuni O:3 lipopolysaccharide and an associated polysaccharide. Eur. J. Biochem. 231:570-578. - PubMed
-
- Aspinall, G. O., A. G. McDonald, H. Pang, L. A. Kurjanczyk, and J. L. Penner. 1994. Lipopolysaccharides of Campylobacter jejuni serotype O:19: structures of core oligosaccharide regions from the serostrain and two bacterial isolates from patients with the Guillain-Barre syndrome. Biochemistry 33:241-249. - PubMed
-
- Avril, T., H. Floyd, F. Lopez, E. Vivier, and P. R. Crocker. 2004. The membrane-proximal immunoreceptor tyrosine-based inhibitory motif is critical for the inhibitory signaling mediated by Siglecs-7 and -9, CD33-related Siglecs expressed on human monocytes and NK cells. J. Immunol. 173:6841-6849. - PubMed
-
- Avril, T., S. D. Freeman, H. Attrill, R. G. Clarke, and P. R. Crocker. 2005. Siglec-5 (CD170) can mediate inhibitory signaling in the absence of immunoreceptor tyrosine-based inhibitory motif phosphorylation. J. Biol. Chem. 280:19843-19851. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

