Alanine esters of enterococcal lipoteichoic acid play a role in biofilm formation and resistance to antimicrobial peptides
- PMID: 16790791
- PMCID: PMC1489678
- DOI: 10.1128/IAI.00111-06
Alanine esters of enterococcal lipoteichoic acid play a role in biofilm formation and resistance to antimicrobial peptides
Abstract
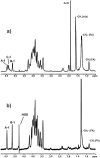
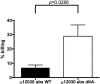
Enterococcus faecalis is among the predominant causes of nosocomial infections. Surface molecules like d-alanine lipoteichoic acid (LTA) perform several functions in gram-positive bacteria, such as maintenance of cationic homeostasis and modulation of autolytic activities. The aim of the present study was to evaluate the effect of d-alanine esters of teichoic acids on biofilm production and adhesion, autolysis, antimicrobial peptide sensitivity, and opsonic killing. A deletion mutant of the dltA gene was created in a clinical E. faecalis isolate. The absence of d-alanine in the LTA of the dltA deletion mutant was confirmed by nuclear magnetic resonance spectroscopy. The wild-type strain and the deletion mutant did not show any significant differences in growth curve, morphology, or autolysis. However, the mutant produced significantly less biofilm when grown in the presence of 1% glucose (51.1% compared to that of the wild type); adhesion to eukaryotic cells was diminished. The mutant absorbed 71.1% of the opsonic antibodies, while absorption with the wild type resulted in a 93.2% reduction in killing. Sensitivity to several cationic antimicrobial peptides (polymyxin B, colistin, and nisin) was considerably increased in the mutant strain, confirming similar results from other studies of gram-positive bacteria. Our data suggest that the absence of d-alanine in LTA plays a role in environmental interactions, probably by modulating the net negative charge of the bacterial cell surface, and therefore it may be involved in the pathogenesis of this organism.
Figures





References
-
- Abachin, E., C. Poyart, E. Pellegrini, E. Milohanic, F. Fiedler, P. Berche, and P. Trieu-Cuot. 2002. Formation of D-alanyl-lipoteichoic acid is required for adhesion and virulence of Listeria monocytogenes. Mol. Microbiol. 43:1-14. - PubMed
-
- Baldassarri, L., L. Bertuccini, R. Creti, P. Filippini, M. G. Ammendolia, S. Koch, J. Huebner, and G. Orefici. 2005. Glycosaminoglycans mediate invasion and survival of Enterococcus faecalis into macrophages. J. Infect. Dis. 191:1253-1262. - PubMed
-
- Baldassarri, L., R. Cecchini, L. Bertuccini, M. G. Ammendolia, F. Iosi, C. R. Arciola, L. Montanaro, R. Di Rosa, G. Gherardi, G. Dicuonzo, G. Orefici, and R. Creti. 2001. Enterococcus spp. produces slime and survives in rat peritoneal macrophages. Med. Microbiol. Immunol. (Berlin) 190:113-120. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

