Identification and characterization of an antigen I/II family protein produced by group A Streptococcus
- PMID: 16790795
- PMCID: PMC1489706
- DOI: 10.1128/IAI.00493-06
Identification and characterization of an antigen I/II family protein produced by group A Streptococcus
Abstract
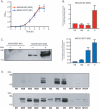
Group A Streptococcus (GAS) is a gram-positive human bacterial pathogen that causes infections ranging in severity from pharyngitis to life-threatening invasive disease, such as necrotizing fasciitis. Serotype M28 strains are consistently isolated from invasive infections, particularly puerperal sepsis, a severe infection that occurs during or after childbirth. We recently sequenced the genome of a serotype M28 GAS strain and discovered a novel 37.4-kb foreign genetic element designated region of difference 2 (RD2). RD2 is similar in gene content and organization to genomic islands found in group B streptococci (GBS), the major cause of neonatal infections. RD2 encodes seven proteins with conventional gram-positive secretion signal sequences, six of which have not been characterized. Herein, we report that one of these six proteins (M28_Spy1325; Spy1325) is a member of the antigen I/II family of cell surface-anchored molecules produced by oral streptococci. PCR and DNA sequence analysis found that Spy1325 is very well conserved in GAS strains of distinct M protein serotypes. As assessed by real-time TaqMan quantitative PCR, the Spy1325 gene was expressed in vitro, and Spy1325 protein was present in culture supernatants and on the GAS cell surface. Western immunoblotting and enzyme-linked immunosorbent assays indicated that Spy1325 was produced by GAS in infected mice and humans. Importantly, the immunization of mice with recombinant Spy1325 fragments conferred protection against GAS-mediated mortality. Similar to other antigen I/II proteins, recombinant Spy1325 bound purified human salivary agglutinin glycoprotein. Spy1325 may represent a shared virulence factor among GAS, GBS, and oral streptococci.
Figures







References
-
- Anonymous. 2005. Active bacterial core surveillance (ABCs) report: emerging infections program network group A Streptococcus, 2004—provisional. [Online.] http://www.cdc.gov/ncidod/dbmd/abcs/survreports/gas04prelim.htm.
-
- Banks, D. J., S. B. Beres, and J. M. Musser. 2005. The contribution of phages to group A Streptococcus genetic diversity and pathogenesis, p. 319-334. In M. K. Waldor, D. I. Friedman, and S. L. Adhya (ed.), Phages: their role in bacterial pathogenesis and biotechnology. ASM Press, Washington, D.C.
-
- Banks, D. J., S. F. Porcella, K. D. Barbian, S. B. Beres, L. E. Philips, J. M. Voyich, F. R. DeLeo, J. M. Martin, G. A. Somerville, and J. M. Musser. 2004. Progress toward characterization of the group A Streptococcus metagenome: complete genome sequence of a macrolide-resistant serotype M6 strain. J. Infect. Dis. 190:727-738. - PubMed
-
- Beres, S. B., G. L. Sylva, K. D. Barbian, B. Lei, J. S. Hoff, N. D. Mammarella, M. Y. Liu, J. C. Smoot, S. F. Porcella, L. D. Parkins, D. S. Campbell, T. M. Smith, J. K. McCormick, D. Y. Leung, P. M. Schlievert, and J. M. Musser. 2002. Genome sequence of a serotype M3 strain of group A Streptococcus: phage-encoded toxins, the high-virulence phenotype, and clone emergence. Proc. Natl. Acad. Sci. USA 99:10078-10083. - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

