Exploitation of the endocytic pathway by Orientia tsutsugamushi in nonprofessional phagocytes
- PMID: 16790799
- PMCID: PMC1489698
- DOI: 10.1128/IAI.01620-05
Exploitation of the endocytic pathway by Orientia tsutsugamushi in nonprofessional phagocytes
Erratum in
- Infect Immun. 2006 Sep;74(9):5422
Abstract
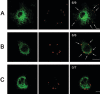
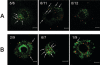
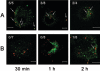
Orientia tsutsugamushi, a causative agent of scrub typhus, is an obligate intracellular bacterium that requires the exploitation of the endocytic pathway in the host cell. We observed the localization of O. tsutsugamushi with clathrin or adaptor protein 2 within 30 min after the infection of nonprofessional phagocytes. We have further confirmed that the infectivity of O. tsutsugamushi is significantly reduced by drugs that block clathrin-mediated endocytosis but not by filipin III, an inhibitor that blocks caveola-mediated endocytosis. In the present study, with a confocal microscope, O. tsutsugamushi was sequentially colocalized with the early and late endosomal markers EEA1 and LAMP2, respectively, within 1 h after infection. The colocalization of O. tsutsugamushi organisms with EEA1 and LAMP2 gradually disappeared until 2 h postinfection, and then free O. tsutsugamushi organisms were found in the cytoplasm. When the acidification of endocytic vesicles was blocked by treating the cells with NH(4)Cl or bafilomycin A, the escape of O. tsutsugamushi organisms from the endocytic pathway was severely impaired, and the infectivity of O. tsutsugamushi was drastically reduced. To our knowledge, this is the first report that the invasion of O. tsutsugamushi is dependent on the clathrin-dependent endocytic pathway and the acidification process of the endocytic vesicles in nonprofessional phagocytes.
Figures
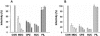
References
-
- Alvarez-Dominguez, C., R. Roberts, and P. D. Stahl. 1997. Internalized Listeria monocytogenes modulates intracellular trafficking and delays maturation of the phagosome. J. Cell Sci. 110:731-743. - PubMed
-
- Beron, W., C. Alvarez-Dominguez, L. Mayorga, and P. D. Stahl. 1995. Membrane trafficking along the phagocytic pathway. Trends Cell Biol. 5:100-104. - PubMed
-
- Caruana, S. 1974. Rickettsial diseases. Trop. Dis. Bull. 71:781-786. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

