Control and regulation of the cellular responses to cold shock: the responses in yeast and mammalian systems
- PMID: 16792527
- PMCID: PMC1513281
- DOI: 10.1042/BJ20060166
Control and regulation of the cellular responses to cold shock: the responses in yeast and mammalian systems
Abstract
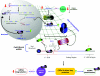
Although the cold-shock response has now been studied in a number of different organisms for several decades, it is only in the last few years that we have begun to understand the molecular mechanisms that govern adaptation to cold stress. Notably, all organisms from prokaryotes to plants and higher eukaryotes respond to cold shock in a comparatively similar manner. The general response of cells to cold stress is the elite and rapid overexpression of a small group of proteins, the so-called CSPs (cold-shock proteins). The most well characterized CSP is CspA, the major CSP expressed in Escherichia coli upon temperature downshift. More recently, a number of reports have shown that exposing yeast or mammalian cells to sub-physiological temperatures (<30 or <37 degrees C respectively) invokes a co-ordinated cellular response involving modulation of transcription, translation, metabolism, the cell cycle and the cell cytoskeleton. In the present review, we summarize the regulation and role of cold-shock genes and proteins in the adaptive response upon decreased temperature with particular reference to yeast and in vitro cultured mammalian cells. Finally, we present an integrated model for the co-ordinated responses required to maintain the viability and integrity of mammalian cells upon mild hypothermic cold shock.
Figures


References
-
- Al-Fageeh M. B., Marchant R. J., Carden M. J., Smales C. M. The cold-shock response in cultured mammalian cells: harnessing the response for the improvement of recombinant protein production. Biotechnol. Bioeng. 2006;93:829–835. - PubMed
-
- Fujita J. Cold shock response in mammalian cells. J. Mol. Microbiol. Biotechnol. 1999;1:243–255. - PubMed
-
- Gualerzi C. O., Giuliodori A. M., Pon C. L. Transcriptional and post-transcriptional control of cold-shock genes. J. Mol. Biol. 2003;331:527–539. - PubMed
-
- Hazel J. R. Thermal adaptation in biological membranes: is homeoviscous adaptation the explanation? Annu. Rev. Physiol. 1995;57:19–42. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

