The CD1d-binding glycolipid alpha-galactosylceramide enhances humoral immunity to T-dependent and T-independent antigen in a CD1d-dependent manner
- PMID: 16792697
- PMCID: PMC1782330
- DOI: 10.1111/j.1365-2567.2006.02413.x
The CD1d-binding glycolipid alpha-galactosylceramide enhances humoral immunity to T-dependent and T-independent antigen in a CD1d-dependent manner
Abstract
Specific interaction of class II/peptide with the T-cell receptor (TCR) expressed by class II-restricted CD4+ T helper (Th) cells is essential for in vivo production of antibodies reactive with T-dependent antigen. In response to stimulation with CD1d-binding glycolipid, Valpha14+ TCR-expressing, CD1d-restricted natural killer T (NKT) cells may provide additional help for antibody production. We tested the hypothesis that the CD1d-binding glycolipid alpha-galactosylceramide (alpha-GC) enhances production of antibodies reactive with T-dependent antigen in vivo. alpha-GC enhanced antibody production in vivo in a CD1d-dependent manner in the presence of class II-restricted Th cells and induced a limited antibody response in Th-deficient mice. alpha-GC also led to alterations in isotype switch, selectively increasing production of immunoglobulin G2b. Further analysis revealed that alpha-GC led to priming of class II-restricted Th cells in vivo. Additionally, we observed that alpha-GC enhanced production of antibodies reactive with T-independent antigen, showing the effects of NKT cells on B cells independently of Th cells. Our data show that NKT cells have multiple effects on the induction of a humoral immune response. We propose that NKT cells could be exploited for the development of novel vaccines where protective antibody is required.
Figures



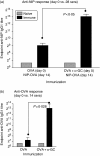

References
-
- McHeyzer-Williams LJ, McHeyzer-Williams MG. Antigen-specific memory B cell development. Annu Rev Immunol. 2005;23:487–513. - PubMed
-
- Slifka MK, Antia R, Whitmire JK, Ahmed R. Humoral immunity due to long-lived plasma cells. Immunity. 1998;8:363–72. - PubMed
-
- Lanzavecchia A. Receptor-mediated antigen uptake and its effect on antigen presentation to class II-restricted T lymphocytes. Annu Rev Immunol. 1990;8:773–93. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

